Refining method for high-purity roflumilast
A technology of roflumilast and a refining method, which is applied in the refining field of high-purity roflumilast, can solve the problem of low yield and the like, and achieve the effects of good appearance, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 roflumilast crude product
[0025] The preparation of roflumilast crude product adopts the document WO09501338 method: 3-(cyclopropylmethoxy)-4-(difluoromethoxy)benzoic acid A is reacted with thionyl chloride to obtain acid chloride B, and then sodium hydrogen Roflumilast is prepared by condensation of base and 3,5-dichloro-4-aminopyridine, and the crude product of roflumilast is obtained, with a general HPLC purity of 98-99%.
[0026] The reaction formula is as follows:
[0027]
[0028] Specifically, the following steps are included:
[0029] 3-(cyclopropylmethoxy)-4-(difluoromethoxy)benzoic acid (A) (282.5g, 1.094mol) was dissolved in toluene (2.8L), and SOCl was added 2 (260.3g, 2.188mol), the external temperature was raised to 110°C for reflux reaction, and a tail gas (HCl) receiving device was added. The reaction was complete after 1 h. Evaporate the solvent and volatile matter (below 90°C) under reduced pressure, add 560ml o...
Embodiment 2
[0033] Add 50g of crude roflumilast and 200ml of ethyl acetate into a 500ml flask, stir, heat to dissolve, cool slightly, add 1.5g of activated carbon, stir and decolorize under reflux for 30min, filter, stir and crystallize the filtrate at room temperature for 10 hours, filter, acetic acid Wash with 20ml of ethyl ester, and dry at 60°C to obtain 35.8g of white crystals, with a yield of 71.6%, a purity of 99.84%, and a single impurity of less than 0.1%.
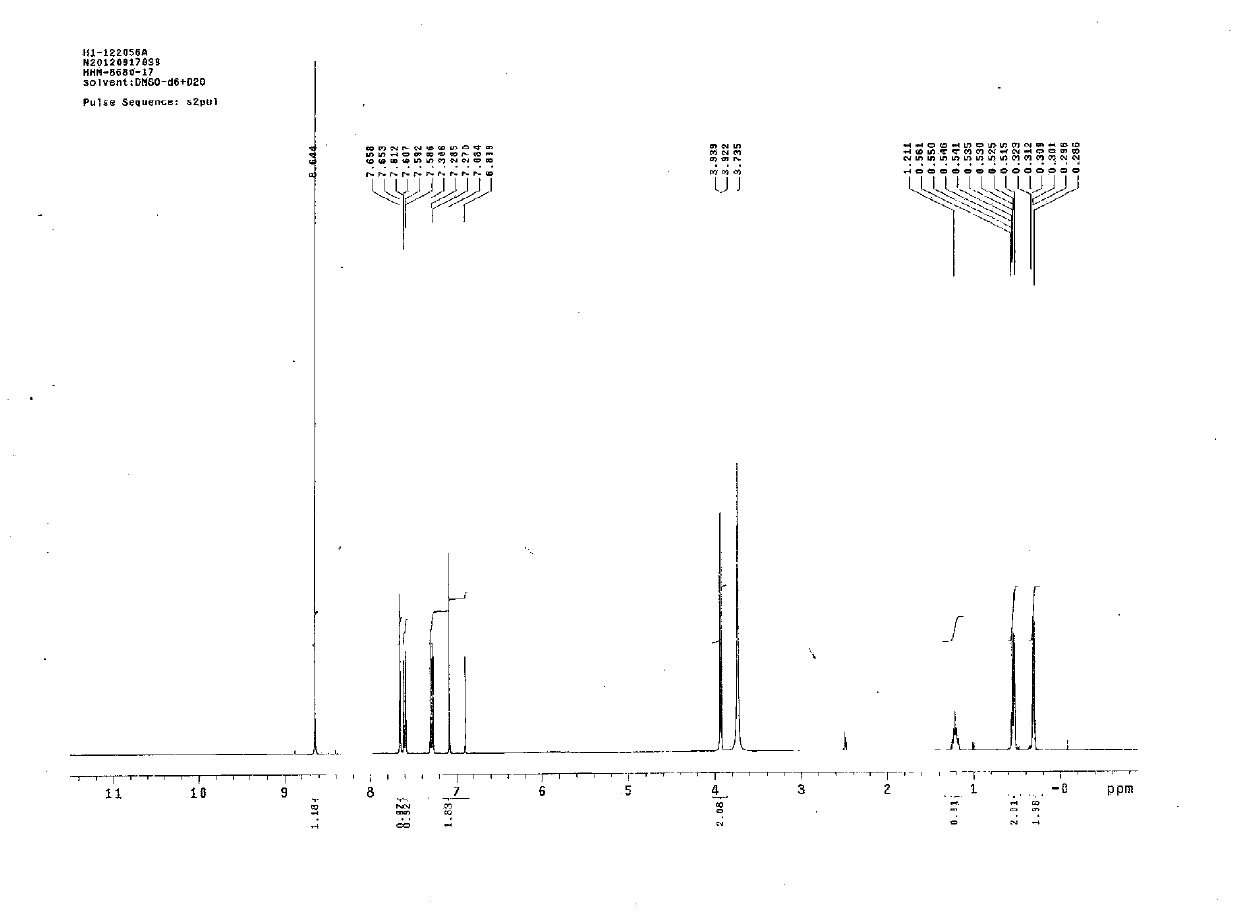
[0034] The roflumilast sample prepared in embodiment 2 was carried out 1 HNMR (δppm) analysis, see figure 1 , 2 , 10.57(s, 1H, heavy water exchange disappeared), 8.73(s, 2H), 7.72(d, 1H, J=1.6Hz), 7.65(dd, 1H, J=1.6, 8.4Hz), 7.35(d, 1H ,J=8.4Hz),7.19(t,1H,J=74Hz),4.00(d,2H,J=7.2),1.29-1.25(m,1H),0.61-0.56(m,2H),0.39-0.35 (m,2H).
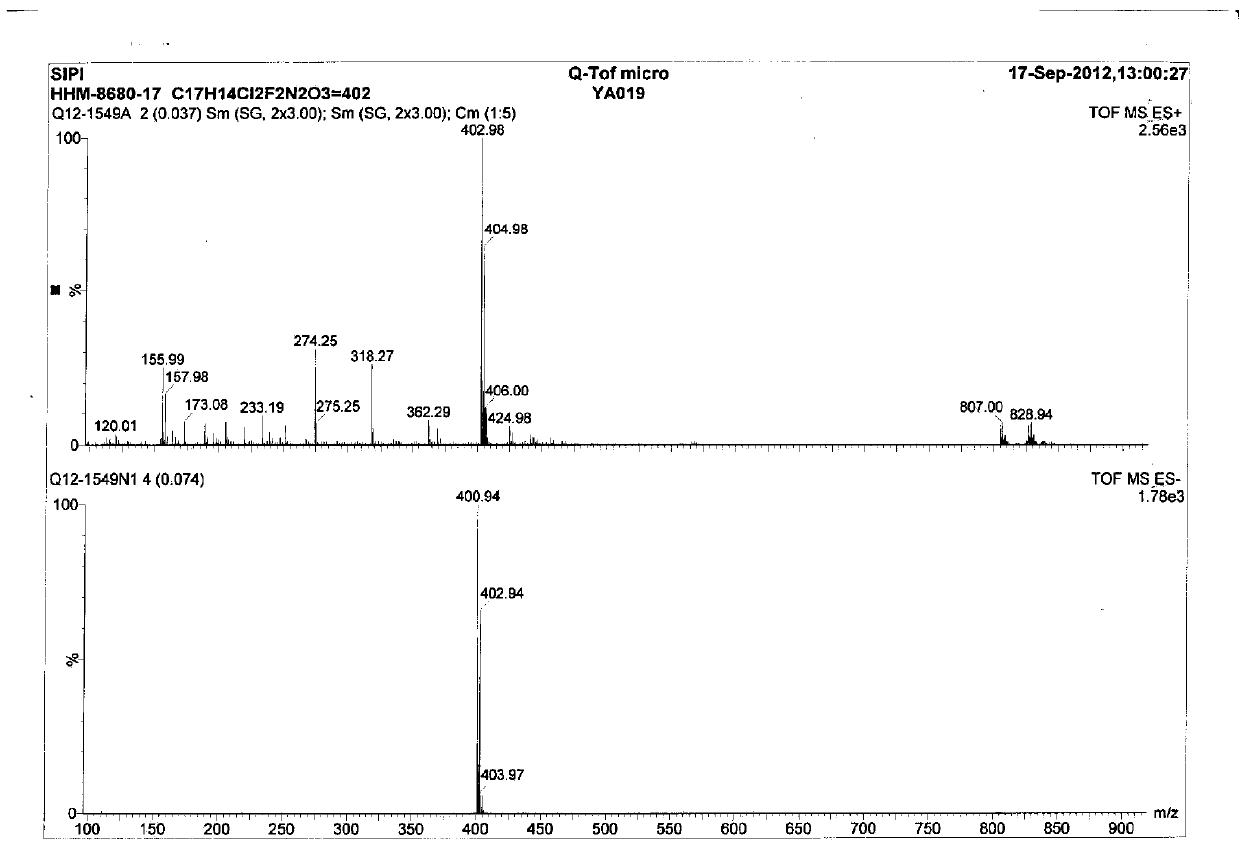
[0035] The roflumilast sample prepared in embodiment 2 is carried out mass spectrometry analysis, see image 3 . ESI + , 402.98 (M+H + ,100%), chlorine isotope peak 404.98 (64%); ESI-, 400.9...
Embodiment 3
[0045] Add 50g of roflumilast crude product and 300ml of acetone into a 500ml flask, stir, heat to dissolve, cool slightly, add 2.5g of activated carbon, stir for decolorization for 30min, filter, stir and crystallize the filtrate at room temperature for 8 hours, filter, wash with 20ml of acetone, 60°C After drying, 37.0 g of white crystals were obtained, with a yield of 74.0%, a purity of 99.86%, and a single impurity of less than 0.1%.
[0046] The roflumilast sample prepared in Example 3 was detected by DSC and X-powder diffraction, and the results were the same as in Comparative Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com