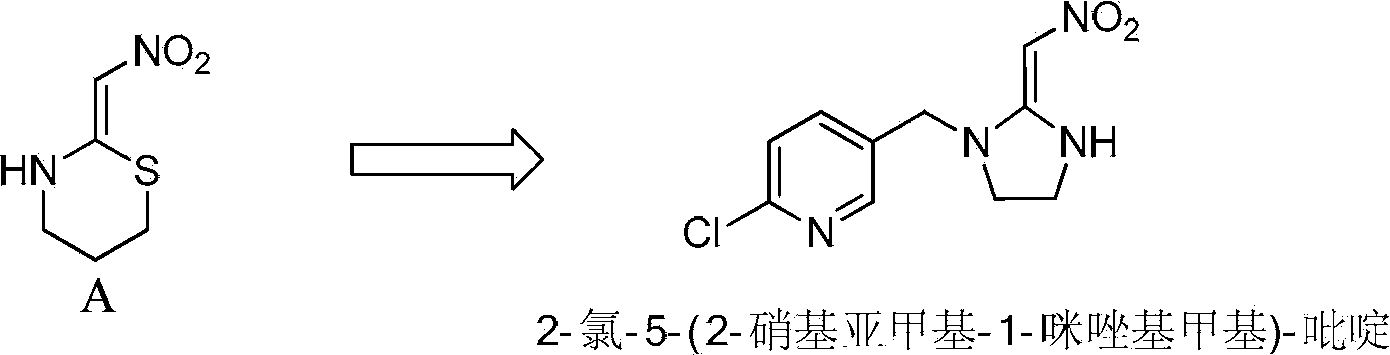
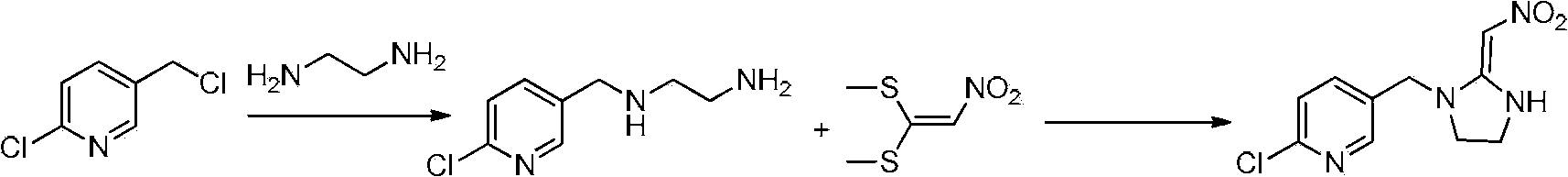Synthesis process of 2-chlorin-5-((2-(nitryl methylene) imidazoline-1-yl) methyl) pyridine
A technology of nitromethylene imidazolidine and nitromethylene is applied in the field of organic compound synthesis, and can solve the problems of poor photostability hindering commercialization, large feed ratio, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
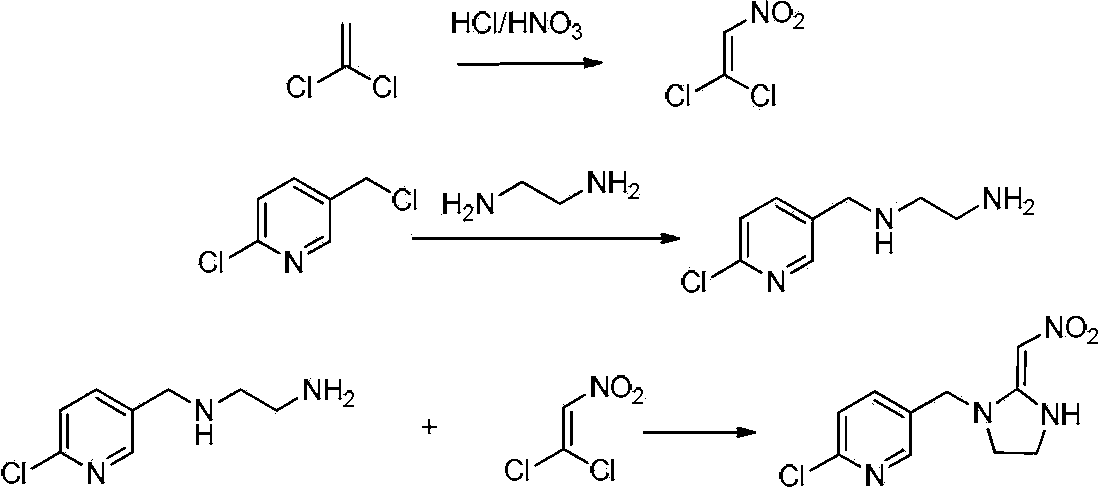
[0110] A preferred method for preparing the intermediate 2-nitromethylene imidazolidine potassium salt, comprising the steps of: (a) using vinylidene chloride as a raw material, and adding vinylidene chloride dropwise to the mixed acid of nitric acid and other acids , to prepare 1,1-dichloro-2-nitroethylene; (b) adding ethylenediamine dropwise to 1,1-dichloro-2-nitroethylene solution to synthesize 2-nitromethylene Imidazolidine; (c) 2-nitromethylene imidazolidine and potassium hydroxide generate 2-nitromethylene imidazolidine potassium salt.
[0111] Generally, the time for dropwise addition of ethylenediamine is 0.5 to 10h, preferably 0.5 to 5h; the holding time after the dropwise addition is 0.5 to 10h, preferably 0.5 to 5h; 2-nitromethyleneimidazolidine The molar ratio to potassium hydroxide is 1:1 to 1:10, preferably 1:5.
[0112] Synthesis of 2-chloro-5-((2-(nitromethylene)imidazolin-1-yl)methyl)pyridine (Process A)
[0113] A synthetic method for synthesizing 2-chloro-...
Embodiment 1
[0181] Embodiment 1. Compound 1, the synthesis of 1-dichloro-2-nitroethylene
[0182]
[0183] A mixture of 41.7g (417mmol) of 36.5% hydrochloric acid and 39.8g (411mmol) of 65% of nitric acid was added dropwise to the reaction solution, and the reaction temperature was controlled at 20~ Between 25°C, add dropwise for 3 hours, keep stirring for 1 hour, wash with water, extract and separate the organic phase with chloroform. Under the condition of stirring in an ice bath, 4% sodium hydroxide solution was added to the chloroform layer to neutralize to pH=7~8, then added chloroform for extraction, combined the extracts, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain 24.6g Yellow-green liquid with a yield of 54.5%.
[0184] GC-MS (m / s): 141(37), 95(85), 83(78), 60(100).
Embodiment 2
[0185] Embodiment 2. The synthesis of compound 2-nitromethylene imidazolidine
[0186]
[0187] 1.175 g (5 mmol) of 1,1-dichloro-2-nitroethylene was dissolved in 10 mL of ethanol and cooled to 0°C. 0.75 g (12.5 mmol) of ethylenediamine dissolved in 10 mL of ethanol was slowly added dropwise to the reaction liquid, the dropping temperature was controlled at 0° C., and the dropping time was 1 h. After the dropwise addition was completed, it was incubated at 0° C. for 1 h. Suction filtration to obtain an orange liquid, wash the filter cake 3 to 4 times with ethanol, combine the filtrates, and concentrate. Recrystallization with ethanol gave 0.37 g of a light brown solid with a yield of 57.1%.
[0188] 1 H NMR (400MHz, DMSO-d6): δ 8.32 (s, 2H), 6.33 (s, 1H), 3.57 (s, 4H) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com