Rotaxane molecular machine of naphthalimide crown ether and pH meter based on rotaxane molecular machine
A naphthalimide crown ether, molecular machine technology, applied in the field of pH meter, can solve the problems of poor repeatability of results, poor test stability, easy to contaminate samples, etc., and achieve the effect of eliminating influence and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
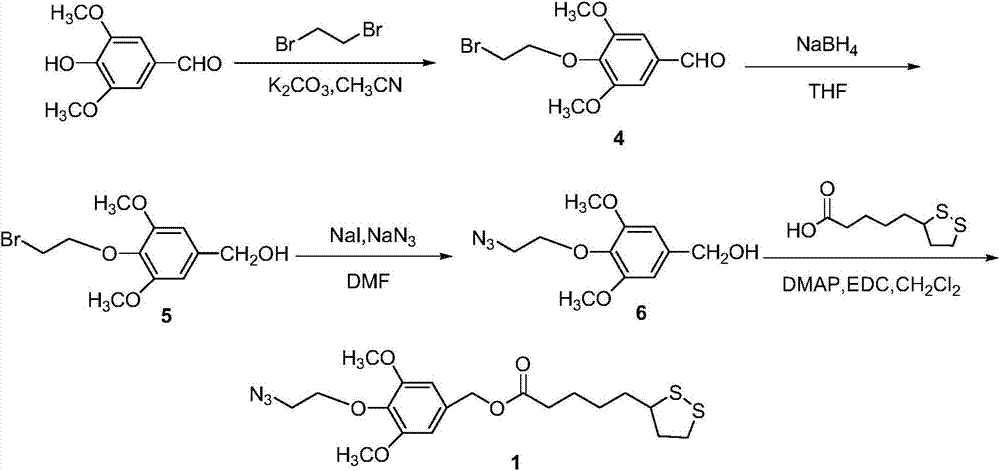
[0035] The disulfide bond-containing azide compound 1 involved in the present invention has a chemical formula of C 19 h 27 N 3 o 5 S 2 , the schematic diagram of its synthetic route is shown in figure 1 As shown, the specific synthesis steps are as follows:
[0036] 1.1 Synthesis of Compound 4
[0037] Add 150mL of acetonitrile, 3,5-dimethoxy-4-hydroxybenzaldehyde (5g, 0.027mol) and 1,2-dibromoethane (19.97g, 0.11mol) into a 250mL round bottom flask, and add Potassium carbonate powder (7.59g, 0.055mol) was heated and refluxed overnight. The reaction solution was poured into 500mL water, stirred for 30min, CH 2 Cl 2 (50mL×3) extraction, anhydrous Na 2 SO 4 After drying, the solvent was removed by column chromatography (SiO 2 , Petroleum ether: dichloromethane = 3: 1), to obtain 6.82 g of pure compound 4 with a yield of 86%. 1 H NMR (CDCl 3 , 400MHz, 298K): δ(ppm)=9.88(s, 1H), 7.13(s, 2H), 4.38-4.33(t, J=8.0Hz, 2H), 3.93(s, 6H), 3.64-3.59( t, J=8.0Hz, 2H).
[003...
Embodiment 2
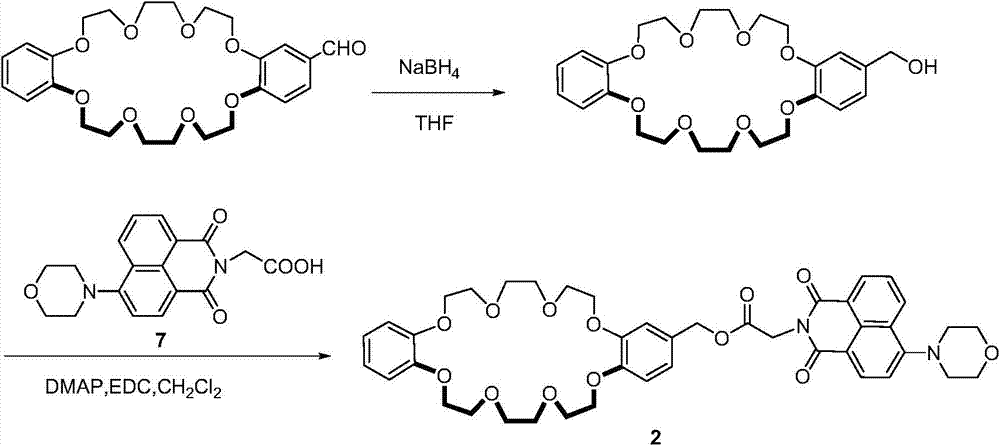
[0045] The present invention relates to naphthalimide crown ether compound 2-naphthoimide dibenzo 24 crown 8 crown ether, and its method route schematic diagram is as follows figure 2 As shown, the specific synthesis steps are as follows:
[0046] 2.1 Synthesis of Compound 2
[0047] Add 25 mL of dry CH to a 50 mL round bottom flask 2 Cl 2 , and then sequentially add such as monoaldehyde dibenzo 24 crown 8 with NaBH 4 After reduction, the reduced product (1 g, 2.09 mmol), compound 7 (0.71 g, 2.09 mmol), DMAP (0.25 g, 2.09 mmol) and EDC·HCl (1.62 g, 8.36 mmol) were stirred overnight under argon protection. The reaction solution was poured into 100mL water and stirred for 30min, CH 2 Cl 2 (25mL×3) extraction, anhydrous Na 2 SO 4 After drying, the solvent was removed by column chromatography (SiO 2 , dichloromethane:methanol=200:1), 1.44g of compound 2 was obtained, and the yield was 86%. 1 H NMR (CDCl 3 , 400MHz, 298K): δ(ppm)=8.60(d, J=4.0Hz, 1H), 8.54(d, J=8.0Hz, 1H...
Embodiment 3
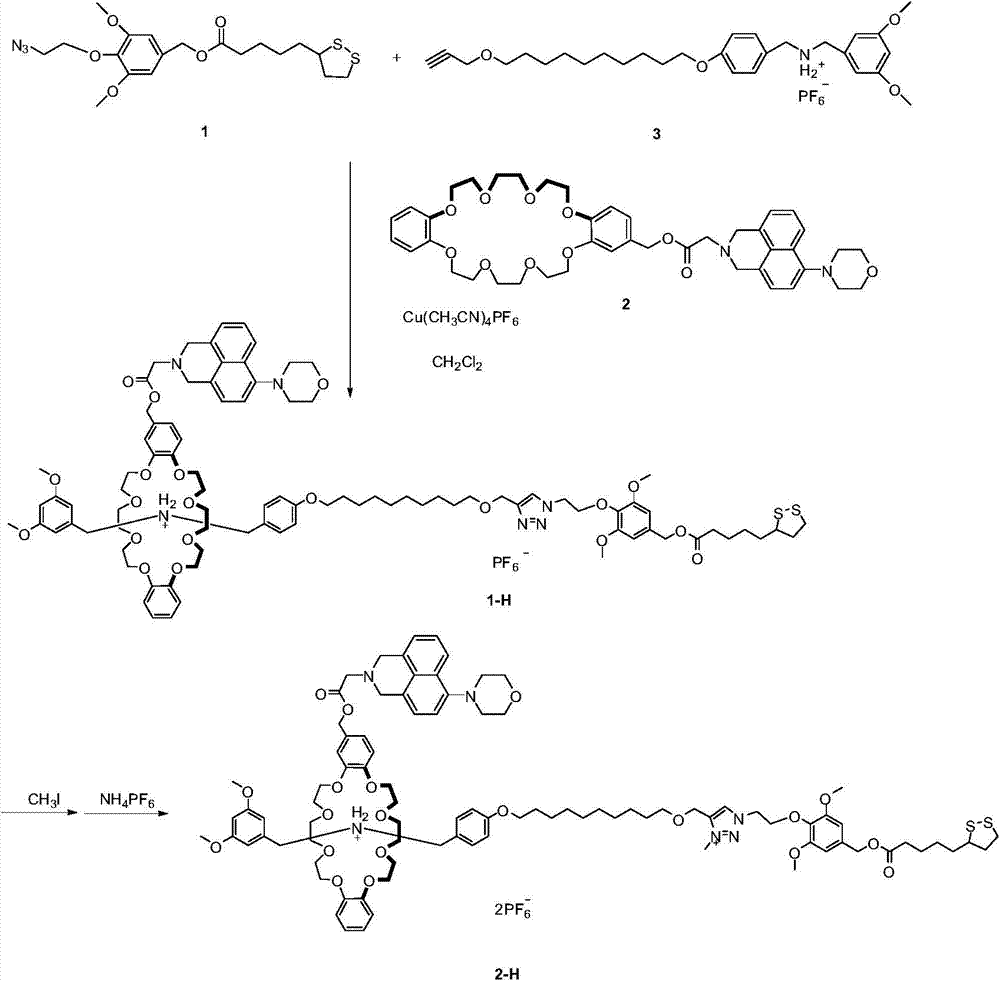
[0049] The schematic diagram of the synthetic route of the rotaxane molecular machine of compound 2-H-naphthalimide crown ether of the present invention is as image 3 As shown, it specifically includes the following steps:
[0050] 3.1 Synthesis of Compound 1-H
[0051] in dry CH 2 Cl 2 Dissolve compound 3 (153mg, 0.249mmol) and naphthalimide crown ether compound 2 (192mg, 0.249mmol) in (10mL), stir at room temperature for 30min, then add disulfide bond-containing azide compound 1 (110mg, 0.249mmol) and [Cu(CH 3 EN) 4 ]PF 6 (93mg, 0.249mmol), stirring was continued for two days. The solvent was spinned off, and column chromatography (SiO 2 , CH 2 Cl 2 : MeOH=100:1) to obtain yellow compound 1-H (50.8 mg, 67%). 1 H NMR (CDCl 3 , 400MHz, 298K): δ(ppm)=8.06(s, 1H), 7.51(s, 2H), 7.18(m, 2H), 7.01(d, J=8.0Hz, 2H), 6.90(m, 2H) , 6.79(m, 2H), 6.70(m, 2H), 6.55(s, 2H), 6.42(s, 2H), 6.24(s, 1H), 5.16(s, 4H), 5.03(s, 2H), 4.80(t, J=4.0Hz, 4H), 4.71-4.66(t, J=4.0Hz, 2H), 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com