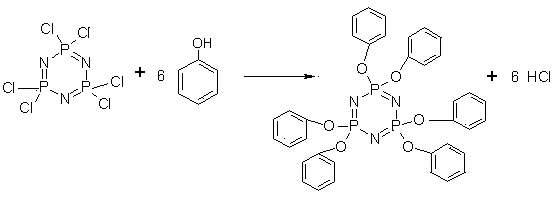
Preparation method of hexaphenoxycyclotriphosphazene
A technology of hexaphenoxycyclotriphosphazene and hexachlorocyclotriphosphazene, which is applied in the field of preparation of hexaphenoxycyclotriphosphazene, can solve the problems of low repeatability, improve product purity, and be easy to realize Effects of industrialization, reduction of raw material consumption and energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of hexachlorocyclotriphosphazene
[0034] A. raw material preparation, prepare raw material according to ammonium chloride 51.2g, composite catalyst 2.56g, pyridine 12.8g, phosphorus pentachloride 256g and chlorobenzene 1024g, wherein catalyzer is the composite catalyst of zinc chloride, ferric chloride, magnesium chloride, its The molar ratio is 1:1:1. Heat in an oil bath, slowly raise the temperature to 120±5°C and reflux, keep the temperature under reflux for 6 to 10 hours, the solution turns from light yellow to light green after a period of reaction, then the reflux temperature rises to 130±5°C until the reaction is completed.
[0035] b. After the reaction, the tap water was cooled to room temperature, and the residue was filtered to obtain a chlorobenzene solution of hexachlorocyclotriphosphazene.
[0036] (2) Preparation of hexaphenoxycyclotriphosphazene
[0037] a. Raw material preparation, the mass ratio of hexachlorocyclotriphosphazene: phen...
Embodiment 2
[0044] (1) Preparation of hexachlorocyclotriphosphazene
[0045] A. raw material preparation, prepare raw material according to ammonium chloride 51.2g, composite catalyst 2.56g, pyridine 12.8g, phosphorus pentachloride 256g and chlorobenzene 1024g, wherein catalyzer is the composite catalyst of zinc chloride, ferric chloride, magnesium chloride, its The molar ratio is 1:1:1. Heat in an oil bath, slowly raise the temperature to 120±5°C and reflux, keep the temperature under reflux for 6 to 10 hours, the solution turns from light yellow to light green after a period of reaction, then the reflux temperature rises to 130±5°C until the reaction is completed.
[0046] b. After the reaction, the tap water was cooled to room temperature, and the residue was filtered to obtain a chlorobenzene solution of hexachlorocyclotriphosphazene.
[0047] (2) Preparation of hexaphenoxycyclotriphosphazene
[0048] a. Raw material preparation, the mass ratio of hexachlorocyclotriphosphazene: phen...
Embodiment 3
[0055] (1) Preparation of hexachlorocyclotriphosphazene
[0056] A. raw material preparation, prepare raw material according to ammonium chloride 51.2g, composite catalyst 2.56g, pyridine 12.8g, phosphorus pentachloride 256g and chlorobenzene 1024g, wherein catalyzer is the composite catalyst of zinc chloride, ferric chloride, magnesium chloride, its The molar ratio is 1:1:1. Heat in an oil bath, slowly raise the temperature to 120±5°C and reflux, keep the temperature under reflux for 6 to 10 hours, the solution turns from light yellow to light green after a period of reaction, then the reflux temperature rises to 130±5°C until the reaction is completed.
[0057] b. After the reaction, the tap water was cooled to room temperature, and the residue was filtered to obtain a chlorobenzene solution of hexachlorocyclotriphosphazene.
[0058] (2) Preparation of hexaphenoxycyclotriphosphazene
[0059] a. Raw material preparation, the mass ratio of hexachlorocyclotriphosphazene: phen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com