Milk oligosaccharide-galactooligosaccharide composition for infant formula containing the soluble oligosaccharide fraction present in milk, and having a low level of monosaccharides, and a process to produce the composition
A galactooligosaccharide, soluble technology, applied in the field of infant formula products, producing the oligosaccharide mixture, to achieve good physical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] The method for preparing the CMOS-GOS mixture of the present invention:
[0151] 207,000 kg whey ultrafiltration permeate was pre-concentrated to 29% (w / w) total solids (TS), pasteurized at about 75°C for about 30 seconds, then concentrated by evaporation at 60°C to reach 58% (w / w) ) of TS. The liquid was divided into 3 crystallizers and each crystallizer was cooled at a rate of 2°C / hour for 24 hours to crystallize the lactose. The crystallized lactose is washed and then removed using a water squeezer. The remaining liquid was clarified with a decanter.
[0152] 114,000 kg of 23% TS material obtained from the clarifier was desalted in a manner known per se through a combination of a weak cation column and a mixed bed column to obtain 109,000 kg of 90% desalted liquid of 14.4% TS.
[0153]5,400 kg of the desalted oligosaccharide mixture was concentrated to 52% TS by evaporation. It was then heated to 60°C in a standard tank at a pH of 5.5 to 6.5. Determine the conce...
Embodiment 2
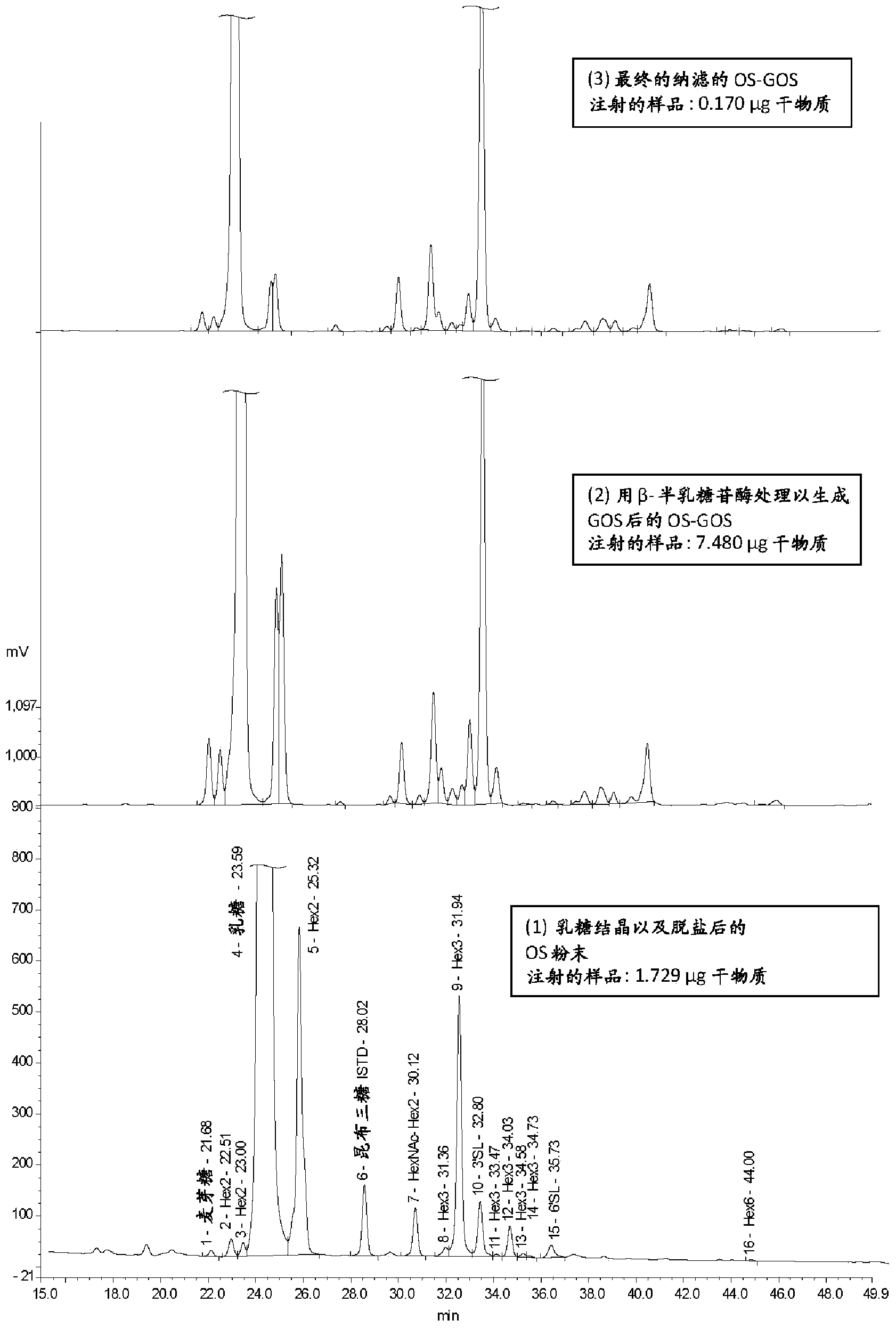
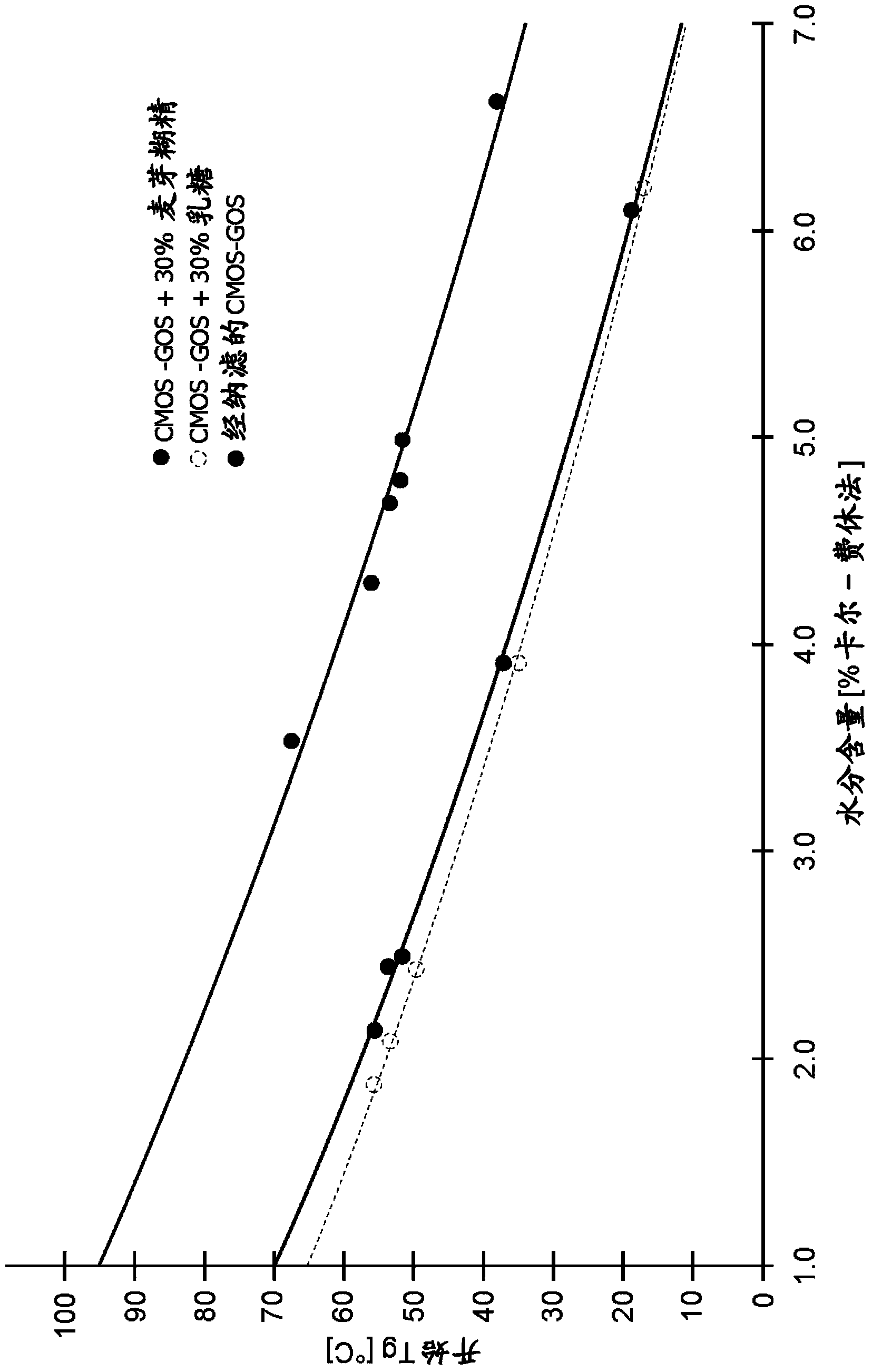
[0160] This example shows the evaluation of total solids oligosaccharides, monosaccharides, ash, lactic acid and citric acid during the process of Example 1 of the invention. CMOS corresponds to the mixture before hydrolysis step d), CMOS-GOS corresponds to the mixture after hydrolysis step d), Nano CMOS-GOS corresponds to the mixture after nanofiltration step f). These samples are also used to generate figure 1 HPLC profiles (1), (2) and (3) in and data in Table 1.
[0161]
Embodiment 3
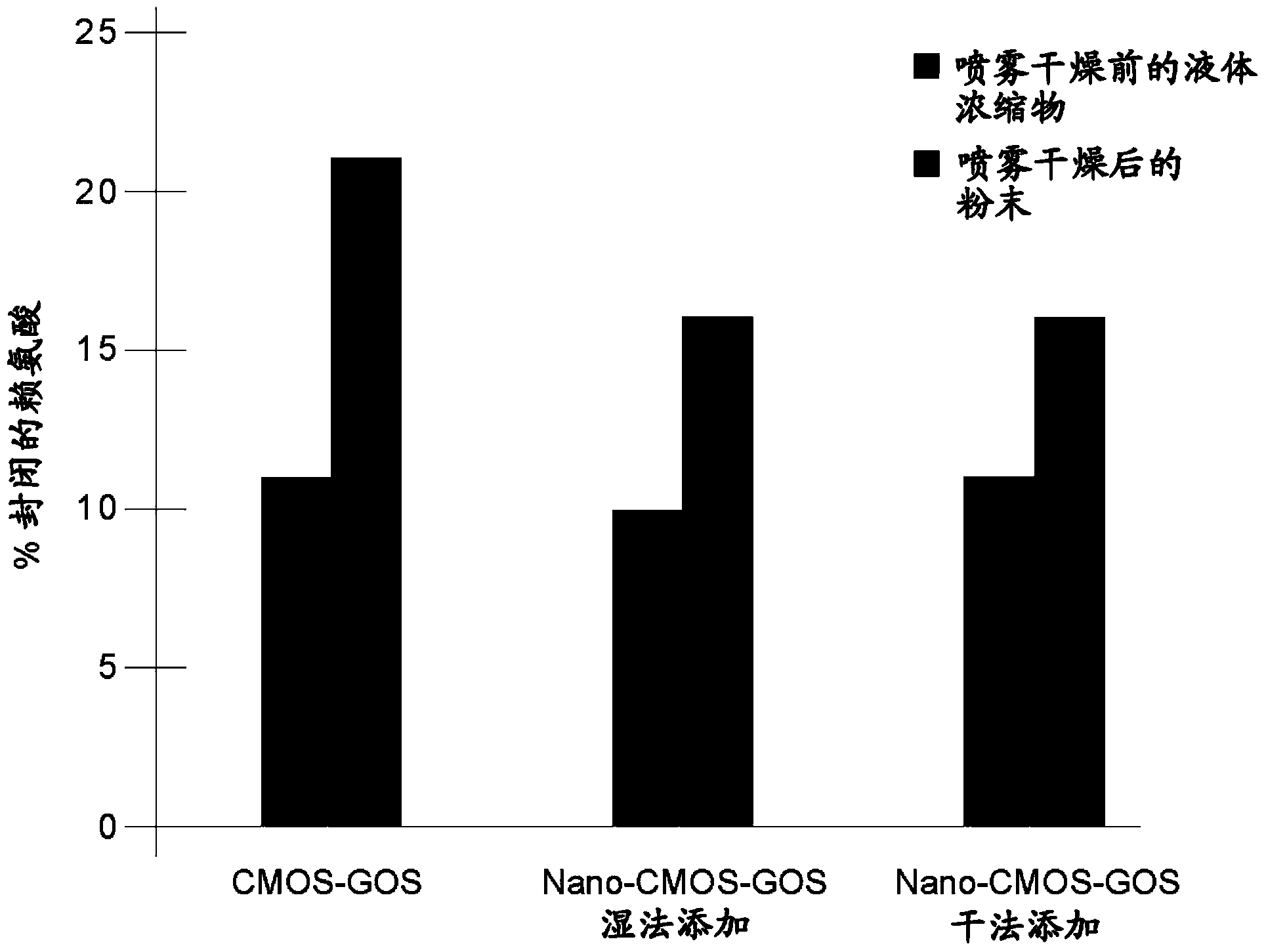
[0163] Decreased Maillard Reactivity After Nanofiltration:
[0164] The infant formulas in Table 2 below were formulated and tested for Maillard activity. In the table, MSW means modified sweet whey, which is a sweet whey from which casein glycomacropeptide (CGMP) has been removed. Infant formulas comprising the CMOS-GOS mixture of the invention, added in the form of a liquid concentrate or as a dry powder, were compared to those comprising non-nanofiltered CMOS and commercially available GOS ( GOS) infant formula was tested against. Standard reaction conditions were used to test Maillard reactivity. The result is as image 3 shown. The oligosaccharide mixtures of the present invention, whether added to infant formula in liquid or powder form, lead to a significant reduction in Maillard reactivity in the final spray-dried product.
[0165] Table 2
[0166]
[0167] 1 long chain polyunsaturated fatty acids
[0168] 2 Example 1
[0169] GOS by FrieslandCampina (N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com