Hapten, artificial antigen and monoclonal antibody for phenothiazine medicaments, preparation methods of hapten and artificial antigen and application of monoclonal antibody
A monoclonal antibody and phenothiazine technology, applied in the field of food safety, can solve the problem that multiple phenothiazine drugs cannot be detected by multi-residue immunoassay, antibodies cannot recognize other phenothiazine drugs at the same time, and there is no cross-reactivity. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of the group-specific hapten of the phenothiazine drug of formula 1 structure
[0029] Experiment 1:
[0030] (a) Add 4mmol 2-chlorophenothiazine into the three-necked flask and dissolve it in 20 mL tetrahydrofuran, add 20mmol sodium hydride under stirring, and stir for 10 minutes;
[0031] (b) Add 4 mmol sodium bromoacetate to the above three-necked flask, heat to reflux, stop heating after 6 hours of reaction and lower to room temperature;
[0032] (c) Add 40 mmol of sodium hydride to the above-mentioned three-necked flask, stop the reaction after 8 hours of reflux reaction and cool down to room temperature;
[0033] (d) Add absolute ethanol to the above-mentioned three-neck flask to remove excess sodium hydride, filter under reduced pressure, and evaporate the obtained filtrate to dryness on a rotary evaporator to obtain a solid, which is acidified in ethanol and separated by column chromatography to obtain Compound shown in formula 1. ...
Embodiment 2
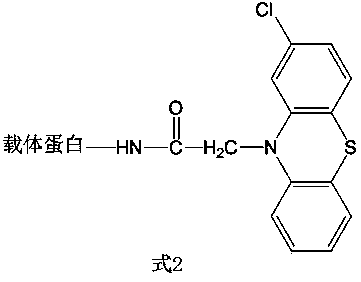
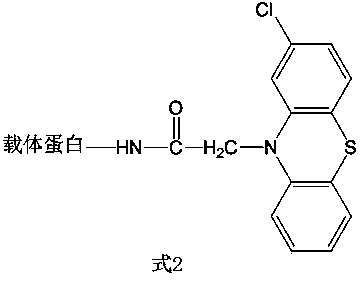
[0044] Example 2: Synthesis of family-specific artificial antigens of phenothiazines with the structure of Formula 2
[0045] Experiment 1:
[0046] (a) Dissolve 0.1 mmol of phenothiazine hapten in 3 mL of N,N-dimethylformamide, then add 20 μL of triethylamine and 0.1 mmol of isobutyl chloroformate, and stir at 4°C for 2 hours. get liquid A;
[0047] (b) Dissolve 0.02 mmol of bovine serum albumin in 2 mL of 1 mol / L sodium bicarbonate solution to obtain liquid B;
[0048] (c) Add liquid A to liquid B dropwise, and stir and react at 4°C for 12 hours to obtain a reaction liquid containing artificial antigens of phenothiazine drugs;
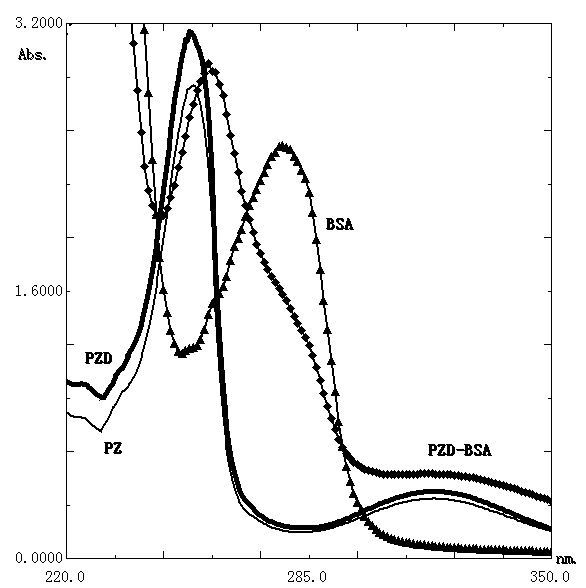
[0049] (d) Dialyze the above-mentioned reaction solution containing the artificial antigen of phenothiazine drugs with PBS at 4°C for 3 days, and filter the solution in the obtained dialysis bag with a filter membrane with a filter diameter of 0.2um under sterile conditions to obtain the following: The artificial antigen of the phenothiazine drugs...
Embodiment 3
[0060] Embodiment 3: the preparation of monoclonal antibody
[0061] (a) Using the artificial antigen of phenothiazine drugs prepared in the above example as the immunogen, immunize 5 Balb / C mice respectively, with an immunization dose of 100-300 μg / mouse, and the immunization method is as follows: mix the immunogen with an equal amount of Freund's complete adjuvant was fully emulsified and injected subcutaneously at multiple points on the back of the neck. At intervals of 2-3 weeks, after emulsifying the immunogen with an equal amount of Freund's incomplete adjuvant, booster immunization once, a total of 6 booster immunizations;
[0062] (b) Seven days after the last immunization, the mouse with the highest serum titer in each group in step (a) above was selected and sacrificed by cervical dislocation. Under sterile conditions, the spleen was removed, splenocytes were isolated, and fused with mouse myeloma SP2 / 0 cells at a ratio of 10:1. Positive hybridoma cells were scree...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com