Terpyridyl-fluorene metal hybrid polymer and electrochromic device containing same
An electrochromic device, terpyridine technology, which is applied in the fields of instrumentation, organic chemistry, optics, etc., can solve the problems of poor dissolution processing performance, difficult to make electrochromic films, etc., achieve good thermal stability, improve solubility, promote The effect of electron cloud delocalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Ligand (7-bis(phenyl-4'-(2,2':6',2"-terpyridine)-9,9-bis(2-ethylhexyl)fluorene ( L1 )Synthesis:
[0036] The synthetic route is as follows:
[0037]
[0038]
[0039] (1) 9,9-bis(2-ethylhexyl)fluorene (compound 2 ) synthesis: Dissolve fluorene (compound 1) (8.48g, 51.1mmol) in anhydrous THF (120ml), stir, and vacuumize the solution and then pass it into N 2 After many times, the solution was cooled to -78°C, and 42.92 ml n-BuLi (107.31 mmol, 2.5M) was slowly added dropwise to the solution. A THF (25ml) solution of 2-ethylhexane (g, 117.53mmol) was slowly added dropwise to the reaction system, the reaction solution was slowly raised to room temperature, and stirred for 3h. Pour the mixture into water, extract with ethyl acetate several times, combine the organic phases, wash the organic phases with saturated brine, separate the liquids, and wash the organic phases with anhydrous NaSO 4 After drying and concentrating, it is separated and purified b...
Embodiment 2
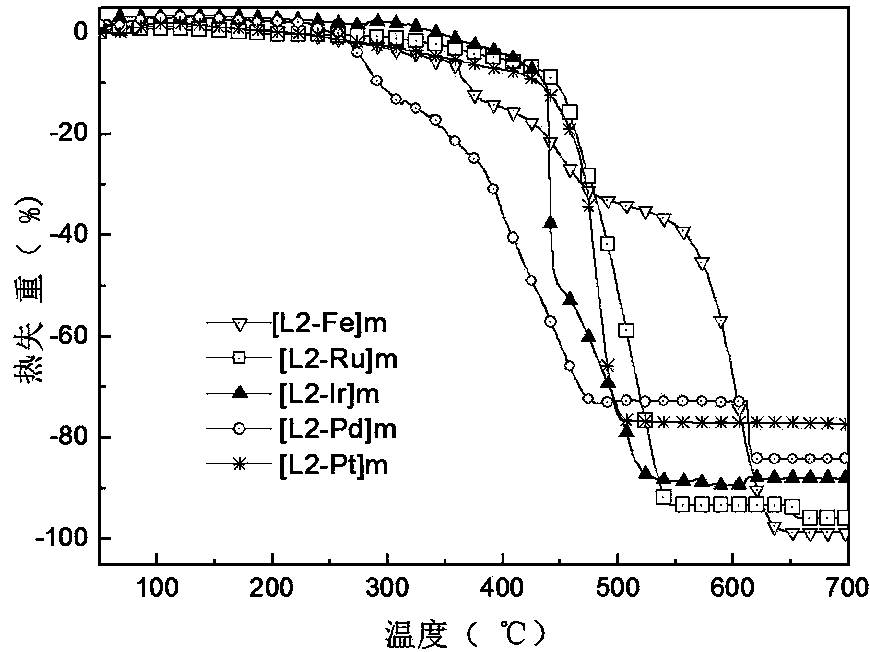
[0047] Example 2 Ligand 7-bis(phenyl-4'-(2,2':6',2"-terpyridine)-9,9-two-dodecylfluorene ( L2 )Synthesis:
[0048] Ligand in synthetic method and embodiment 1 The synthesis method is the same, only need to change the 1-bromo-2-ethylhexane in the step (1) of Example 1 to n-dodecyl bromide. The resulting product is the ligand 2, Expressed as L2, the yield is 71.8%, the melting point is 67°C, and its molecular formula is as follows:
[0049]
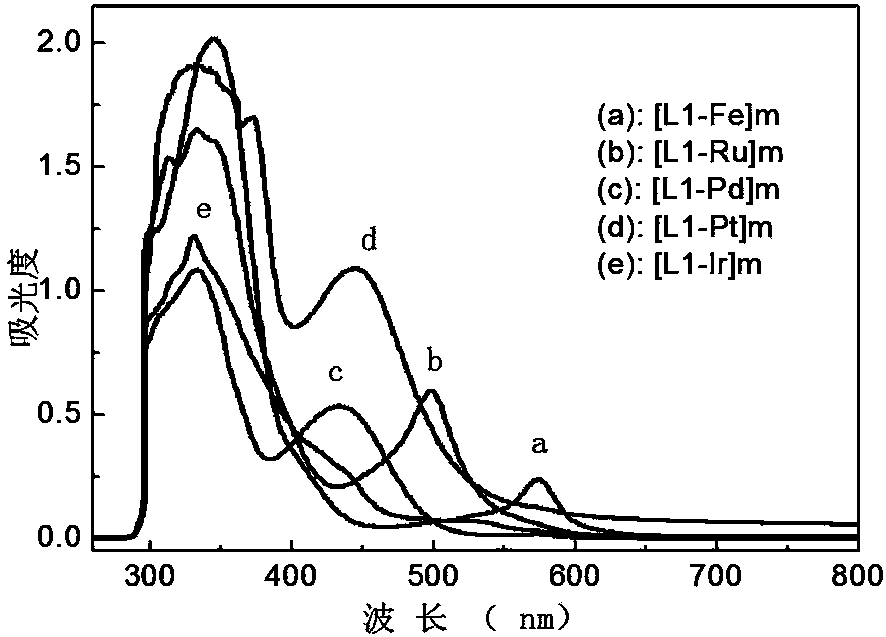
[0050] UV absorption peak position: λ ab =350 nm (ε=6′10 4 cm -1 × M -1 , CH 2 Cl 2 solvent); 1 H NMR (400 MHz, CDCl 3 , TMS): δ 0.80, 0.82, 0.83 (t, 6H, -CH 3 ), 1.10, 1.19, 1.24 (t, 40 H, -CH 2 -), 2.09, 2.11, 2.13 (t, 4H, -CH 2-), 7.36, 7.38, 7.39 (t, 4H, pyr-H), 7.66, 7.68, 7.70 (t, 4H, Ar-H), 7.83, 7.85 (d, 6H, fluorene-H), 7.88, 7.90, 7.92 (t, 4H, Ar-H), 8.04, 8.06 (d, 4H, pyr-H), 8.69, 8.71 (d, 4H, pyr-H), 8.76, 8.77 (d, 4H, pyr-H) , 8.83 (s, 4H, pyr-H).
Embodiment 3
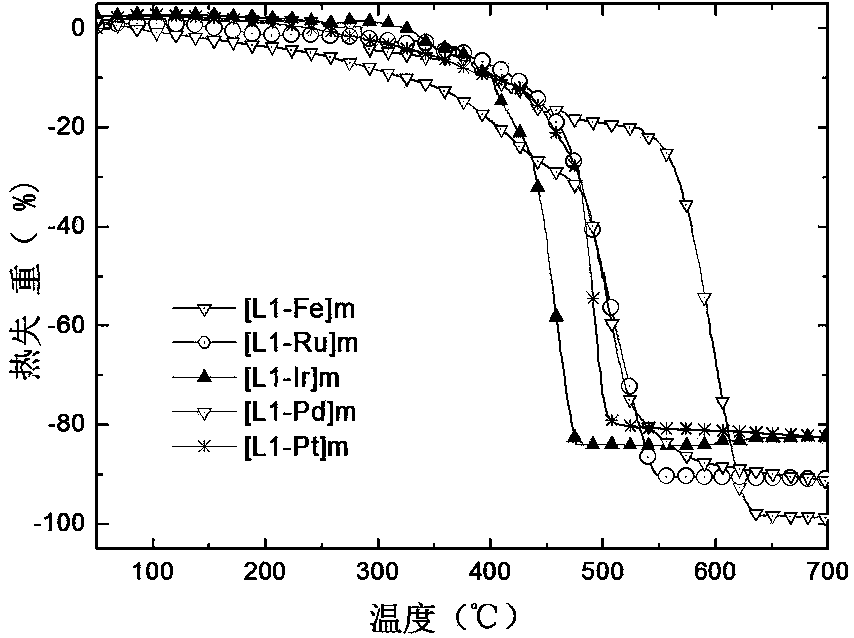
[0051] Example three terpyridine-fluorene iron hybrid polymer ([L1-Fe] m ) preparation:
[0052] At room temperature, add L1 (0.0475 g, 0.05 mmol) and Fe 2 SO 4 Fe 2 SO 4 Ammonium hexafluorophosphate with 10 times the amount of substance, continue to stir for 0.5h, filter with suction, wash with absolute ethanol and absolute ethanol / acetonitrile, and dry in a vacuum oven to obtain the product [L1-Fe] m . Yield: 85%. The melting point is greater than 400°C, and the critical decomposition temperature is 332°C. Structural Characterization Data: 1 H NMR (400 MHz, DMSO, TMS): δ 0.593 (s, 12H, -CH 3 ), 0.858 (s, 18H, alkyl hydrogen), 2.232 (s, 4H, -CH 2 -),7.242 (s, 8H, Ar-H),7.577 (s, 4H, Ar-H),8.087(s, 6H, fluorene-H), 8.246(s, 4H, pyr-H),8.721, 8.738 (s, 4H, pyr-H), 8.810, 8.824(s, 4H, pyr-H), 9.147 (s, 4H, pyr-H), 9.794(s, 4H, pyr-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com