A kind of photodynamic bactericidal polymer and its preparation method and application
A polymer, photodynamic technology, applied in photodynamic therapy, chemical instruments and methods, antibacterial drugs, etc., can solve the problems of limited sterilization range, general sterilization effect, inability to kill fungi, etc., and achieves simple preparation method and strong killing. Ability, low concentration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 polymer (I)
[0027] 1) In a 100mL round bottom flask, add 2.68g 2,5-dibromohydroquinone (10.0mmol), 32.8g 1,12-dibromododecane (100mmol), 4.97g potassium carbonate (36.0 mmol) and 100mL acetone. Under the catalytic amount of 18-crown-6 phase transfer catalyst, stirring and heating under reflux for 5h. Stop the reaction, spin off a large amount of solvent under reduced pressure, add 200 mL of dichloromethane, wash the obtained organic phase with distilled water 3 times (3×100 mL), dry over anhydrous sodium sulfate, and remove the solvent to obtain a crude product. The crude product was separated by column chromatography (eluent: dichloromethane / petroleum ether=1 / 2, v / v) to obtain 1,4-dibromo-2,5-bis(12-bromododecyloxy)benzene (II) 2.68 g (35.1% yield). 1 H NMR (400MHz, CDCl 3 ,ppm)δ:1.23-1.49(m,32H),1.77-1.89(m,8H),3.36(t,JJ=7.2Hz,4H),3.94(t,JJ=8.0Hz,4H),7.08(s ,2H); 13 C NMR (100MHz, CDCl 3 , ppm) δ: 25.88, 28.15, 28.68, 29.14, 29...
Embodiment 2
[0030] The test of the fluorescence emission spectrum and the ultraviolet absorption spectrum of embodiment 2 polymer (I)
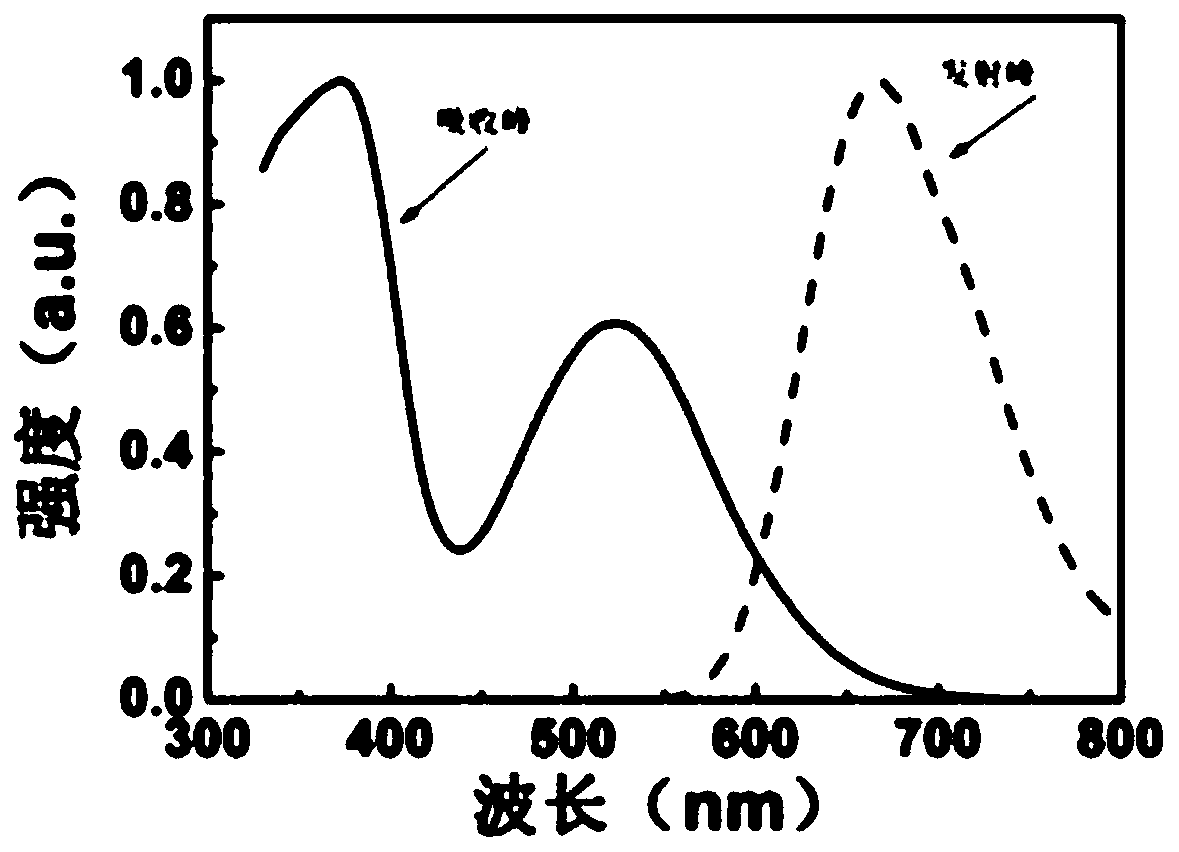
[0031] Polymer (I) was dissolved in DMSO to prepare a solution with a concentration of 0.02 mg / mL. Accurately pipette 2.0mL of the above solution into the UV sample cell, and use DMSO solvent as a reference, measure it on a HITACHI UH5300 UV absorber, and the obtained maximum absorption peak is 540nm. Also accurately pipette the above-mentioned DMSO solution with a concentration of 0.02mg / mL and add it to a 2.0mL fluorescence sample cell, measure it on a HITACHI F-4600 fluorescence instrument, the excitation and emission slit width is 5n, the excitation wavelength is 513nm, and the obtained maximum emission wavelength 665nm. The normalized spectrum of the obtained results can be seen in figure 1 .
Embodiment 3
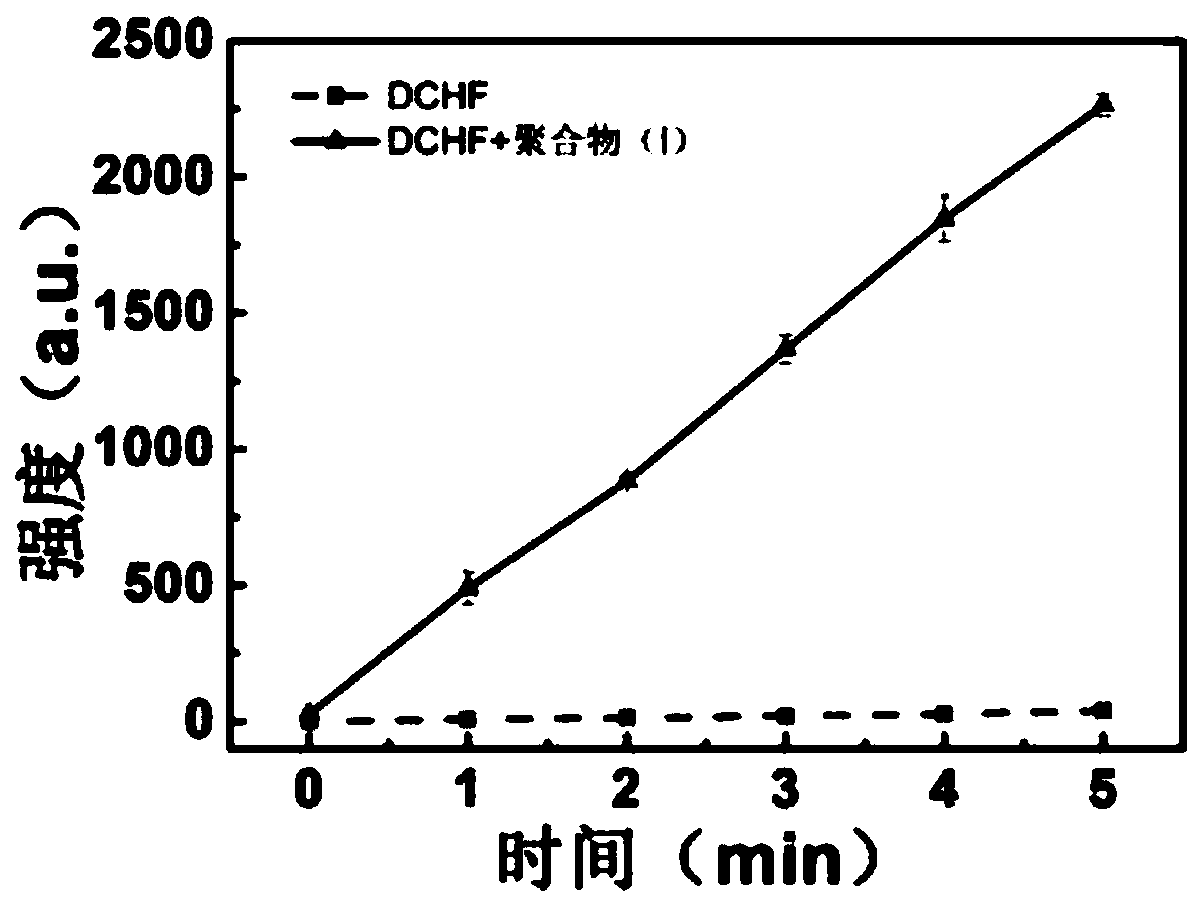
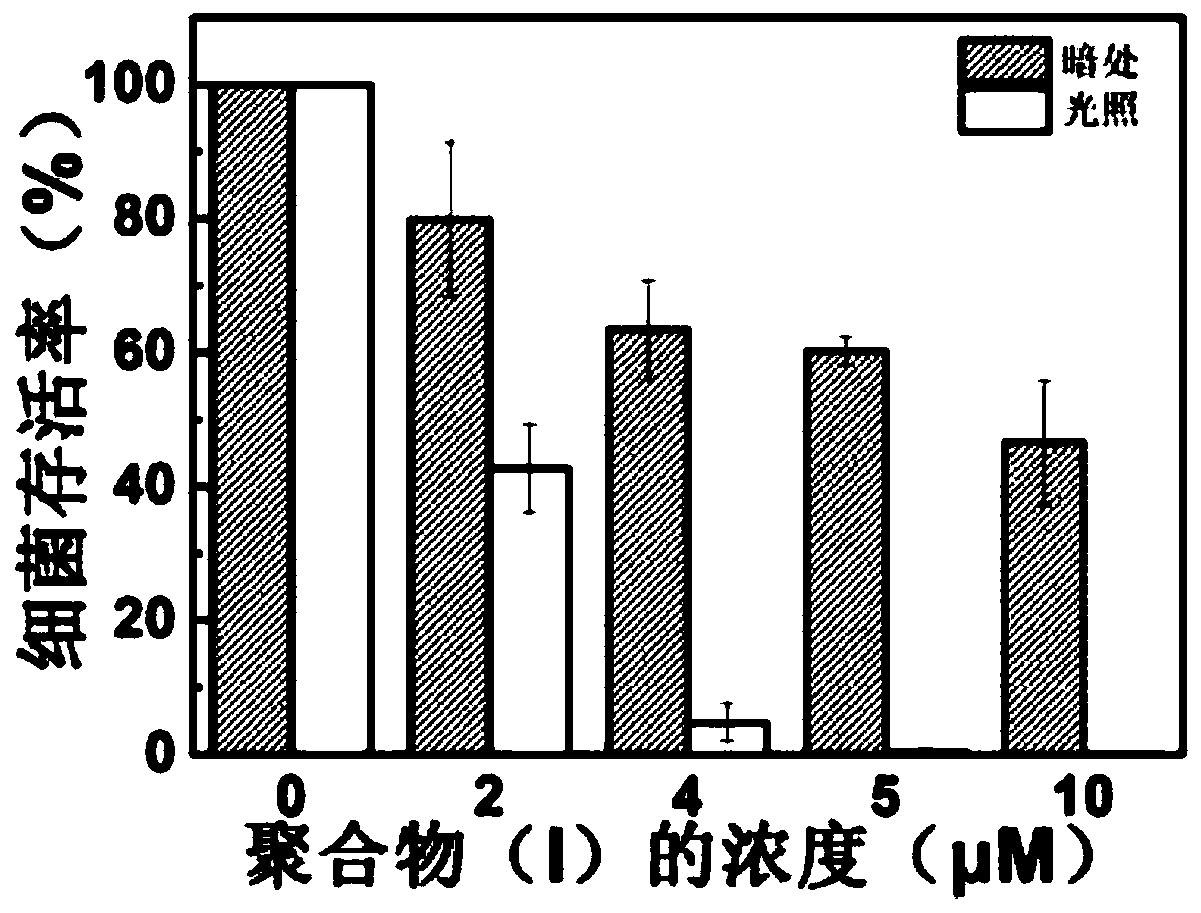
[0032] The active oxygen generation ability test of embodiment 3 polymer (I)
[0033] Take 50 μL of 10.0 mM ethanol solution of 2,7-dichlorofluorescein diacetate, add 450 μL of ethanol to dilute, then add 2.0 mL of 0.01M NaOH aqueous solution, and activate at room temperature for 30 min in the dark. After activation, 10 mL of 1×PBS buffer solution was added, and the final concentration of the mixed DCFH solution was 40 μM.
[0034] Add 1.0 mL of activated DCFH (40 μM) solution and 52.4 μL of polymer (I) (100 μM) aqueous solution into a cuvette, mix well, and expose the resulting solution under white light (1.0 mW / cm 2 ) under irradiation for 5 minutes, record the fluorescence emission spectrum of the DCFH solution with an excitation wavelength of 488nm at 500-700nm every minute, and the blank group is the DCFH solution (40 μ M) without adding any activated DCFH to be tested. After the same light treatment, use the same method to detect its fluorescence emission spectrum. For...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com