Double-membrane filtration method for microbiological testing of pharmaceuticals
A technology of microbiological testing and filtering method, applied in the field of drug microbiological testing, can solve the problems of patients' health and life safety threats, the test cannot be continued, and microbial contamination, etc., and achieve the effect of simple and feasible method, effective results, and elimination of bacteriostatic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
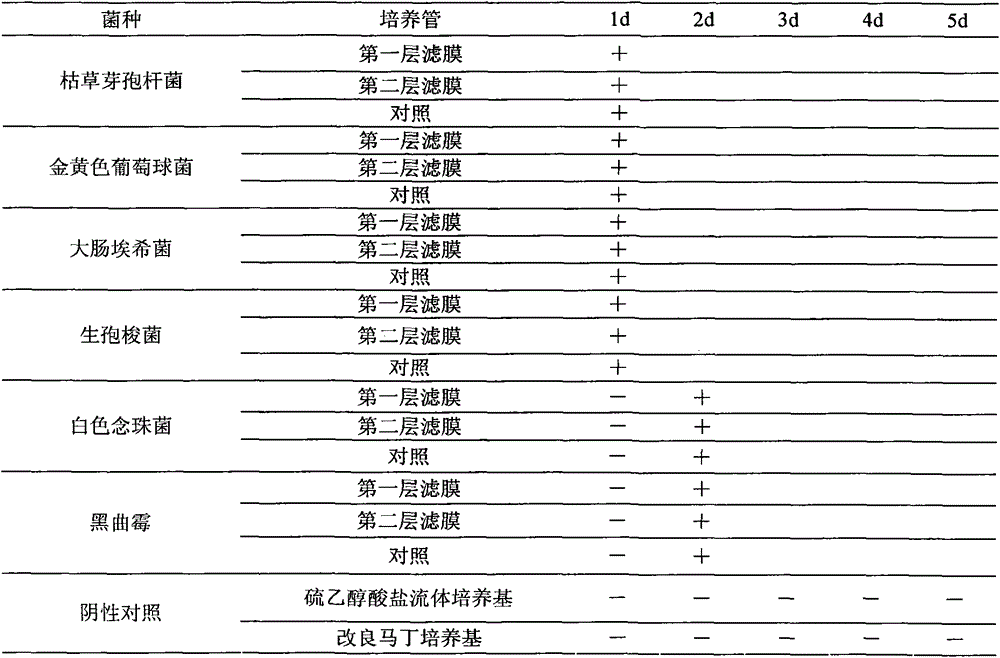
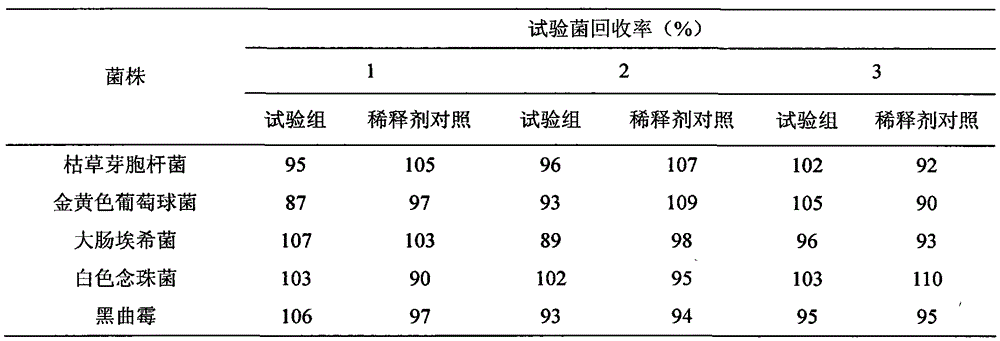
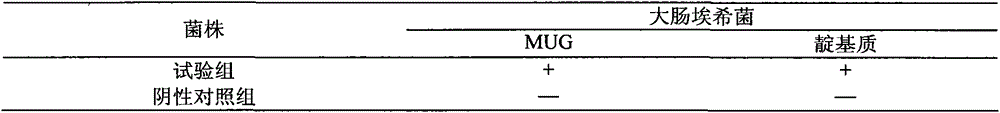
[0030] Take the sterility test of ganciclovir ophthalmic gel as an example.
[0031] 1. Medium and reagents: thioglycolate fluid medium, modified Martin medium, nutrient broth medium, nutrient agar medium, rose bengal sodium agar medium, 0.1% peptone buffer, anhydrous calcium chloride.
[0032] 2. Test sample: Ganciclovir ophthalmic gel.
[0033] 3. Experimental strains: Bacillus subtilis [CMCC (B) 63501], Escherichia coli [CMCC (B) 44102], Staphylococcus aureus [CMCC (B) 26003]; Clostridium sporogenes [CMCC (B) 64941], Candida albicans [CMCC(F)98001], and Aspergillus niger [CMCC(F)98003] were all provided by the China Institute for Food and Drug Identification.
[0034] 4. Bacterial solution preparation: Escherichia coli, Staphylococcus aureus, and Bacillus subtilis use nutrient broth medium, Clostridium sporogenes use thioglycolate fluid medium, culture at 30-35°C for 18-24 hours; white Candida adopts modified Martin's medium and cultures at 20-25°C for 24-48 hours; Asperg...
Embodiment 2
[0044] Take the microbial limit test of compound berberine tablets as an example.
[0045] 1. Instruments BSC-1500IIA2-X biological clean safety cabinet; MJ-250I mold incubator, GHP-9270 water-proof constant temperature incubator; SartoriusCP225D electronic balance; YT-X301 microbial limit tester.
[0046] 2. The test product compound Andrographis lotus tablets.
[0047]3. Bacillus subtilis (Bacillus subtilis) [CMCC (B) 63501]; Escherichia coli (Escherichiacoli) [CMCC (B) 44102]; Staphylococcus aureus (Staphylococcus aureus) [CMCC (B) 26003]; Candida albicans Candida albicans [CMCC (F) 98001]; Aspergillus niger (Aspergillus niger) [CMCC (F) 98003] are all from the Medical Culture Collection Center of China Institute for the Control of Pharmaceutical and Biological Products. .
[0048] 4. Culture medium Nutrient agar medium, lot number 101021; rose bengal sodium agar medium, lot number 1001122pH7.0 sodium chloride-peptone buffer, lot number 110421; all purchased from Beijing ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com