Preparation method for zinc oxide/stannic oxide composite microsphere
A technology of tin dioxide and composite microspheres, applied in the directions of zinc oxide/zinc hydroxide, microsphere preparation, tin oxide, etc., can solve the problems of poor selectivity, lack of synthesis methods, etc., and achieve easy components and sizes, and simple methods. , the effect of uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
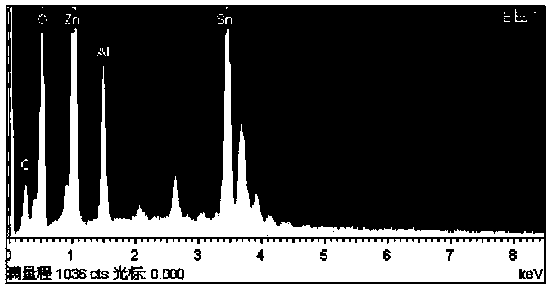
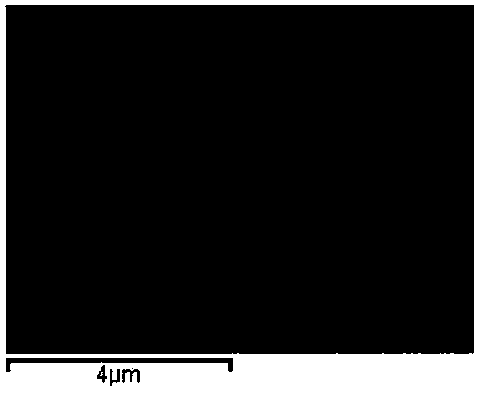
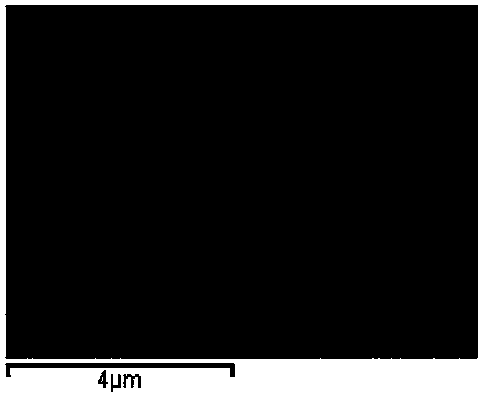
Image
Examples
Embodiment 1
[0024] ZnO / SnO 2 Preparation of composite precursor solution:
[0025] Weigh 2.1 g of triblock copolymer polyoxyethylene-polyoxypropylene-polyoxyethylene (P123), dissolve the block polymer in absolute ethanol, stir, and after P123 is completely dissolved, add 0.2 g of stannous chloride water, stirred at room temperature for 3 hours; then weighed 0.9 g of zinc acetate dihydrate, stirred at room temperature for 3 hours.
[0026] ZnO / SnO 2 Preparation of composite microspheres:
[0027] The prepared ZnO / SnO 2 The composite precursor solution was placed in a polytetrafluoroethylene autoclave, reacted at 180°C for 24 hours, and cooled naturally to room temperature. Filtered, washed with absolute ethanol and deionized water several times, dried at 60 °C, and fully ground to obtain the final product ZnO / SnO 2 Composite microspheres.
Embodiment 2
[0029] ZnO / SnO 2 Preparation of composite precursor solution:
[0030] Weigh 2.1 g of triblock copolymer polyoxyethylene-polyoxypropylene-polyoxyethylene (P123), dissolve the block polymer in absolute ethanol, stir, and after P123 is completely dissolved, add 0.2 g of stannous chloride water, stirred at room temperature for 3 hours; then weighed 0.9 g of zinc acetate dihydrate, stirred at room temperature for 3 hours.
[0031] ZnO / SnO 2 Preparation of composite microspheres:
[0032] The prepared ZnO / SnO 2 The composite precursor solution was placed in a polytetrafluoroethylene autoclave, reacted at 200°C for 24 hours, and cooled naturally to room temperature. Filtered, washed with absolute ethanol and deionized water several times, dried at 60 °C, and fully ground to obtain the final product ZnO / SnO 2 Composite microspheres.
Embodiment 3
[0034] ZnO / SnO 2 Preparation of composite precursor solution:
[0035] Weigh 2.1 g of triblock copolymer polyoxyethylene-polyoxypropylene-polyoxyethylene (P123), dissolve the block polymer in absolute ethanol, stir, and after P123 is completely dissolved, add 0.1 g of stannous chloride water, stirred at room temperature for 3 hours; then weighed 0.45 g of zinc acetate dihydrate, stirred at room temperature for 3 hours.
[0036] ZnO / SnO 2 Preparation of composite microspheres:
[0037] The prepared ZnO / SnO 2 The composite precursor solution was placed in a polytetrafluoroethylene autoclave, reacted at 120°C for 24 hours, and cooled naturally to room temperature. Filtered, washed with absolute ethanol and deionized water several times, dried at 80 °C, and fully ground to obtain the final product ZnO / SnO 2 Composite microspheres.
[0038] Example 3:
[0039] ZnO / SnO 2 Preparation of composite precursor solution:
[0040] Weigh 2.1 g of triblock copolymer polyoxyethylene-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com