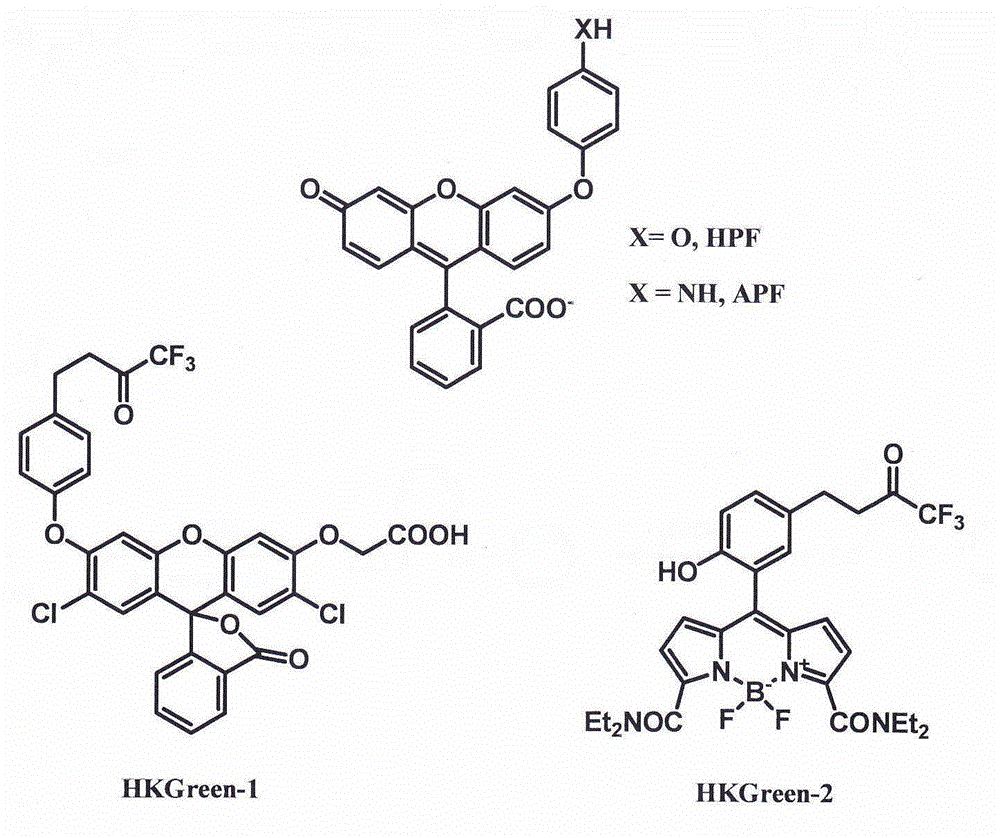
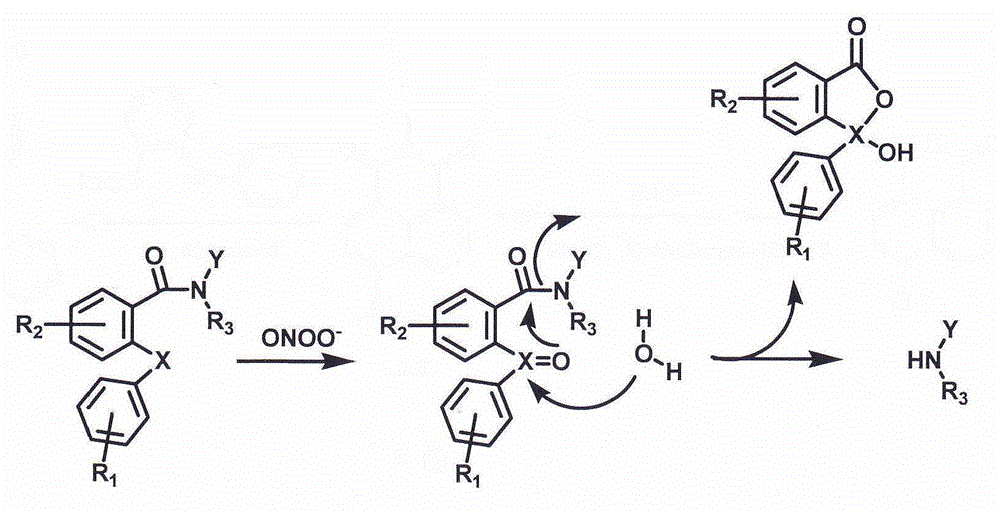
A fluorescent probe for detecting nitrosyl peroxide by cleavage of amide bond and its application
A technology of peroxynitrosyl and fluorescent probes, applied in the field of fluorescent probes, can solve problems such as inability to achieve quantitative detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 (synthesis of intermediate compound BOD):
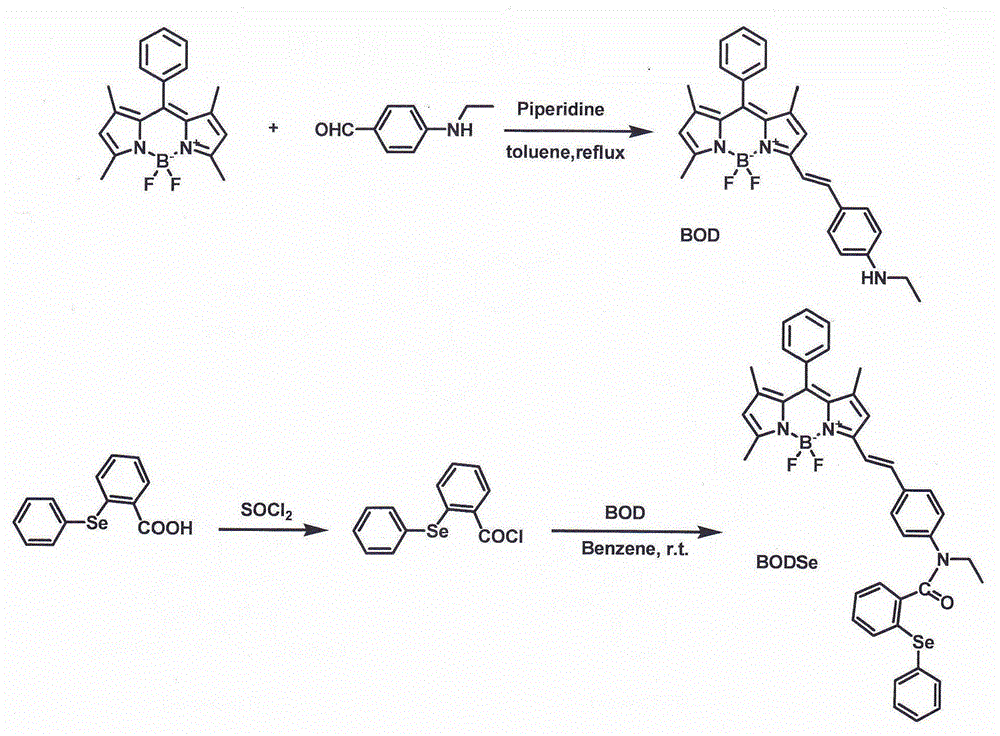
[0059] Such as image 3 As shown, the structure of the probe compound used in the examples is represented by the code BODSe, and the dye matrix used in the synthesis of the probe compound is represented by the code BOD.
[0060] Synthesis of BOD: raw material BODIPY (J.Phys.Chem.A 1998,102,10211-10220) 0.76g, p-ethylaminobenzaldehyde (JOC 2011,76,1180-1183) 0.3g, 1mL piperidine as catalyst, 20mL of o-dichlorobenzene was used as a solvent, protected by nitrogen, reacted at 140°C for 6h, and evaporated to dryness. Purify by column separation, collect the red fluorescent components, and evaporate the solvent to obtain a green shiny solid.
[0061] 1 H NMR (400MHz, CDCl 3 -D 1 )δ(ppm):7.49-7.17(m,8H),6.58-6.56(s,2H),5.96(s,2H),3.90(s,2H),3.22-3.20(q,2H),2.58(s ,3H),1.54(s,3H),1.41-1.37(s,3H),1.27-1.26(t,3H).GC-MS(API-ES):m / z C 28 h 28 BF 2 N 3 Calcd 455.2, found 455.2.
Embodiment 2
[0062] Embodiment 2 (synthesis of probe BODSe):
[0063] Synthesis of probe BODSe: Dissolve 0.3g of o-phenylseleniumbenzoic acid (Organic Syntheses Coll.1988, 6,533, J.Chem.Soc., Perkin Trans.1999,1, 1915-1916) and 2mL of thionyl chloride in 5mL In dichloromethane, react at 65°C for 7 h, evaporate the solvent to dryness under reduced pressure, and dissolve the obtained solid with 10 mL of benzene. The acid chloride solution was dropped into a solution formed by dissolving 0.41 g of BOD in 10 mL of benzene, stirred at room temperature for 10 hours, and evaporated to dryness. The obtained solid was purified by column chromatography to obtain the target compound BODSe.
[0064] 1 H NMR (400MHz, CDCl 3 -D 1 )δ(ppm):7.60-7.29(m,8H),7.16-6.96(m,12H), 6.55(s,1H),6.03(s,1H),4.10-3.99(q,2H),2.60(s ,3H),1.59(s,3H),1.42-1.39(d,3H),1.27-1.26(m,3H). 13 CNMR (100MHz, CDCl 3 -D 1 )δ(ppm):182.25,168.70,153.79,151.82,143.62,142.94,141.93,141.54,141.37,139.41,135.62,132.36,131.69,131.62,...
Embodiment 3
[0065] Embodiment 3 (synthesis of intermediate compound NA):
[0066] Such as Figure 4 As shown, the structure of the probe compound used in the examples is represented by the code NASe, and the dye precursor used in the synthesis of the probe compound is represented by the code NA.
[0067] 2.77g of 4-bromo-1,8-naphthoic anhydride, 5mL of 40% methylamine aqueous solution, 20mL of ethylene glycol monomethyl ether, reacted at 135°C for 18h under the protection of nitrogen, poured the reaction liquid into 200mL of water, dichloromethane Extract until the aqueous layer is colorless, combine the organic layers, and dry over anhydrous sodium sulfate. Purified by column separation to obtain 1.8 g of yellow powder.
[0068] 1 H NMR (400MHz, CDCl 3 ,ppm)δ8.45(d,1H,J=8.0Hz),8.30(d,1H,J=8.0Hz),8.25(d,1H,J=8.4Hz),7.92(t,1H,J=8.0 Hz),7.17(d,1H,J=8.4Hz),4.76(s,1H),3.62(s,3H),3.52(s,3H).
[0069] GC-MS(API-ES): m / z C 14 h 12 N 2 o 2 [M+H] + Calcd: 241.0899, found: 241.0932.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com