Improved method for preparing exenatide sustained release microspheres
A technology for exenatide and sustained-release microspheres, which is applied in the field of improved preparation of exenatide sustained-release microspheres, which can solve the problems of reduced polymer dissolution rate, long dissolution time, and exacerbated polypeptide denaturation, achieving a production cycle The effect of shortening, accelerating the dissolution rate, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of Exenatide Sustained Release Microspheres by Ordinary Single Emulsion Solvent Evaporation Method
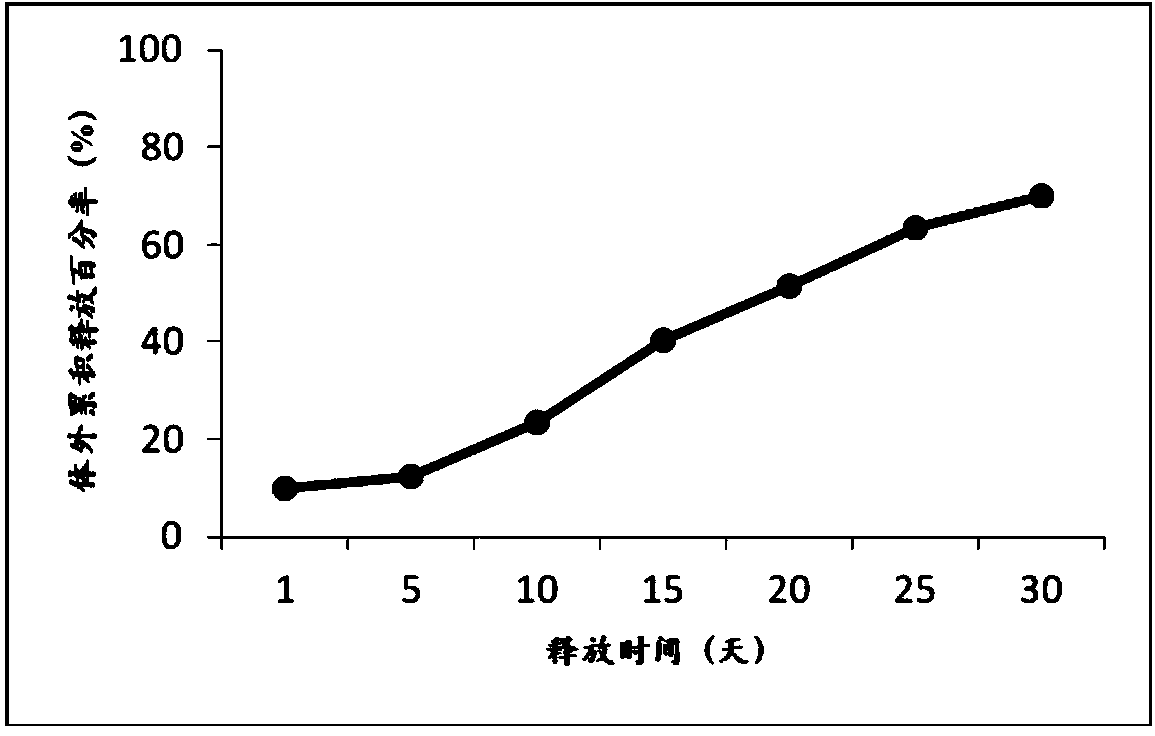
[0035] Weigh 0.75 g of exenatide and dissolve it in 75 mL of methanol by ultrasonication for 30 min. Weigh 24g of poly(lactic-co-glycolic acid) copolymer (75 / 25, 0.2dL / g) and dissolve it in 75mL of dichloromethane. Vortex and mix methanol and dichloromethane for 10s to form a clear solution, pour it into 15L of 1% polyvinyl alcohol aqueous solution under mechanical stirring to obtain double emulsion, keep stirring the double emulsion for more than 3 hours to remove the organic solvent, centrifuge and wash , the microspheres were collected, the average particle size of the microspheres was about 34 μm, the drug loading capacity was 2.60%, the encapsulation efficiency was 85.9%, and the total impurity content was 3.04%.
Embodiment 2
[0036] Example 2: Preparation of Exenatide Sustained Release Microspheres by Improved Single Emulsion Solvent Evaporation Method
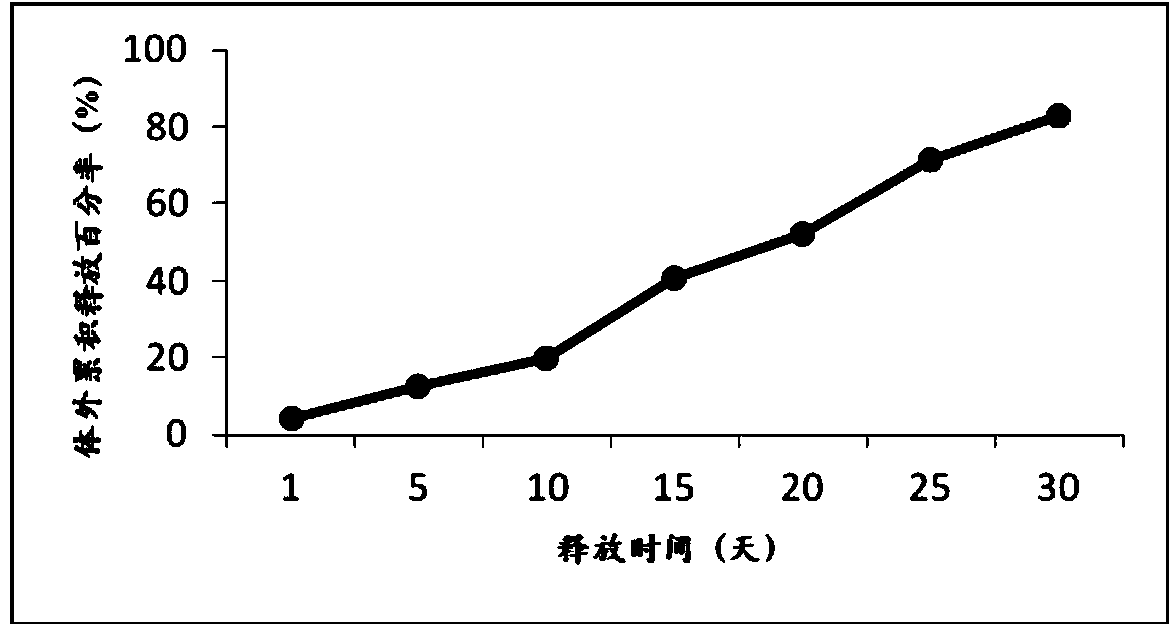
[0037] Weigh 24g of poly(lactic-co-glycolic acid) copolymer (75 / 25, 0.2dL / g) and dissolve it in 75mL of dichloromethane, add 75mL of methanol, vortex the mixed solution for 10s to form a clear solution, weigh 2.00g of exenatide , add the mixed solution, vortex for 10s to form a clear solution, pour it into 15L of 1% polyvinyl alcohol aqueous solution under mechanical stirring to obtain double emulsion, keep stirring the double emulsion for more than 3 hours to volatilize and remove the organic solvent, centrifuge, wash, and collect to obtain Microspheres, the average particle size of the microspheres is about 39 μm, the drug loading capacity is 6.65%, the encapsulation efficiency is 86.4%, and the total impurity content is 0.55%.
Embodiment 3
[0038] Example 3: Preparation of Exenatide Sustained Release Microspheres by Improved Single Emulsion Solvent Evaporation Method
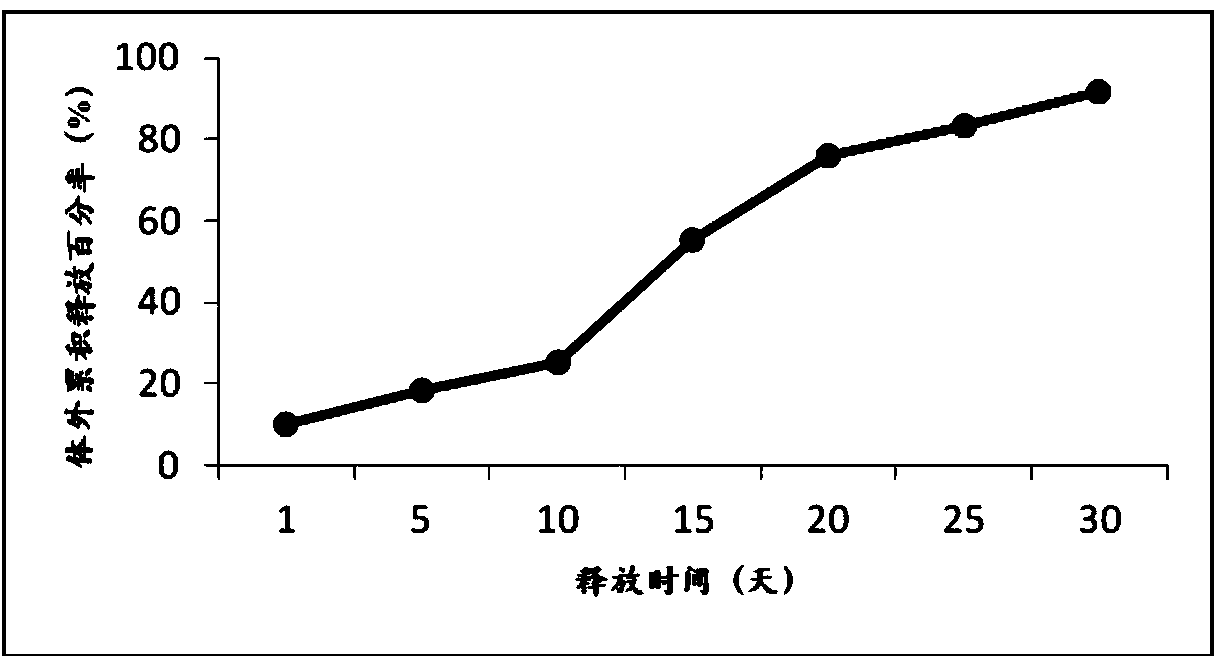
[0039] Weigh 24g of poly(lactic-co-glycolic acid) copolymer (50 / 50, 0.2dL / g) and dissolve it in 100mL of dichloromethane, add 50mL of methanol, vortex the mixed solution for 10s to form a clear solution, weigh 2.00g of exenatide and micronized zinc acetate 1.50g, add the mixed solution, vortex to form a suspension, inject it into 15L of 0.5% polyvinyl alcohol and 5% sodium chloride aqueous solution under mechanical stirring to obtain double emulsion, and make this compound The milk was continuously stirred for more than 3 hours to volatilize the organic solvent, centrifuged, washed, and collected to obtain microspheres. The average particle size of the microspheres was about 41 μm, the drug loading was 6.94%, the encapsulation efficiency was 90.2%, and the total impurity amount was 0.98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com