Celecoxib solid composition with increased dissolution rate, and preparation method and application thereof
A technology of solid composition and celecoxib, which is applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of affecting the therapeutic effect of drugs, affecting solubility, and dissolution effects. To achieve satisfactory results and other issues, to achieve the same chemical properties and celecoxib monomer, to improve the effect of slow dissolution and good dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
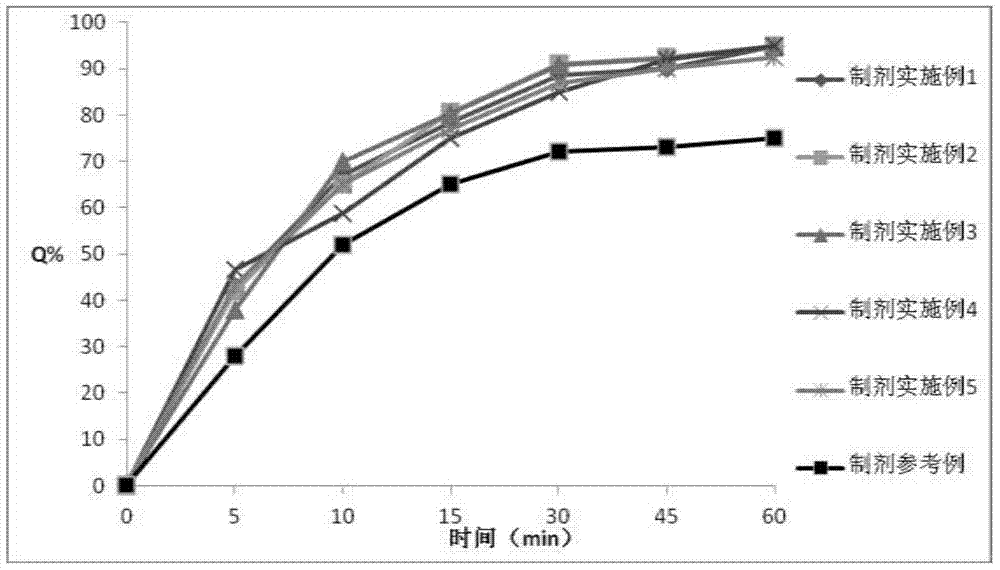
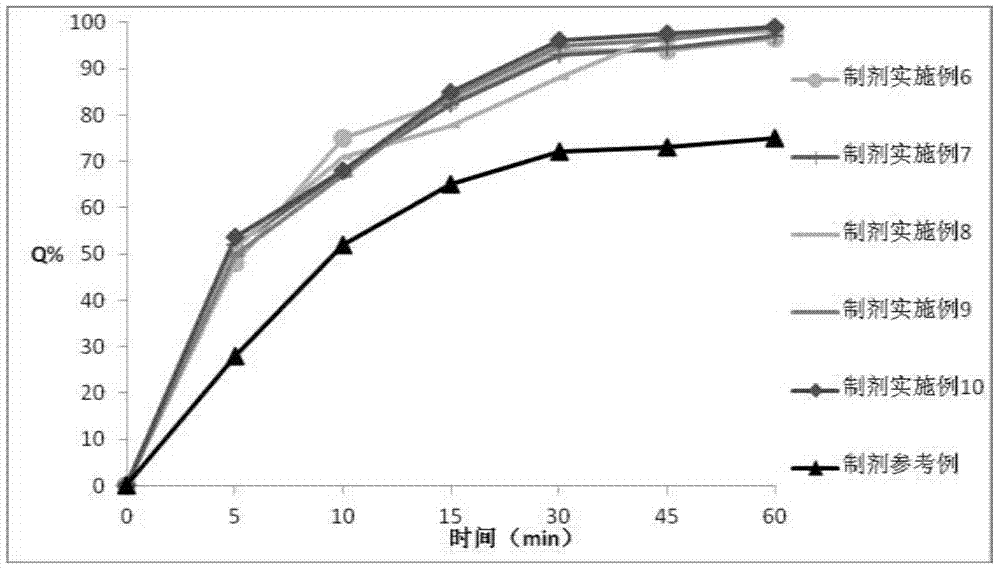
Examples
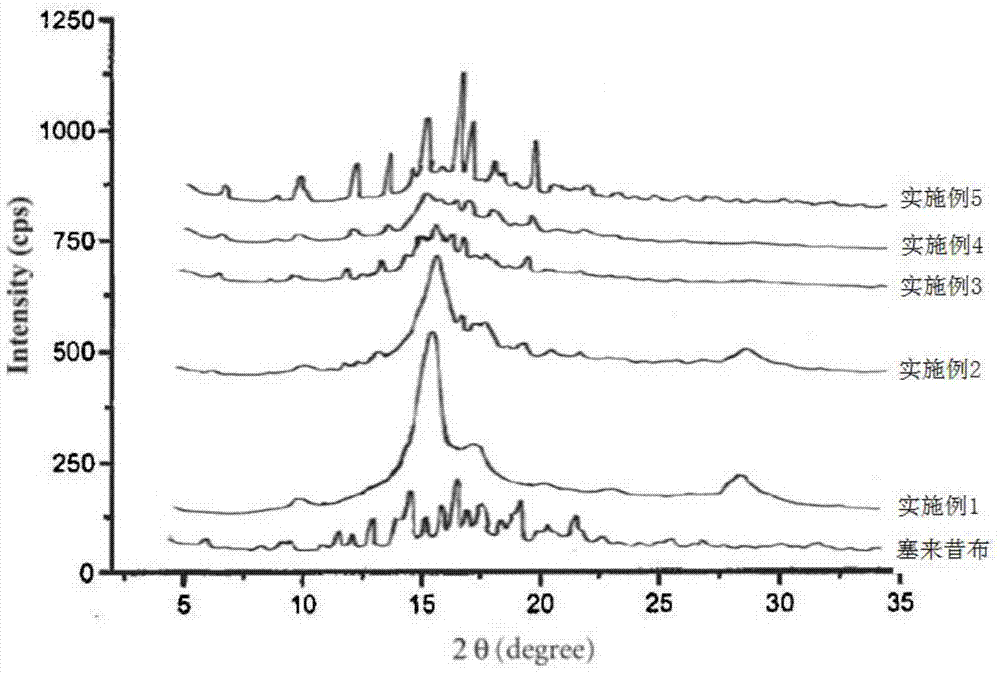
Embodiment 1
[0041] Embodiment 1, solid composition
[0042] Take celecoxib 100g, polyvinylpyrrolidone K 30 45g, 40g of hydroxypropyl methylcellulose, 1.0g of disodium hydrogen phosphate and 0.5g of sodium hydroxide (of which polyvinylpyrrolidone K 30 and hydroxypropyl methylcellulose as a dispersing co-solvent, disodium hydrogen phosphate and sodium hydroxide as an alkaline compound), mix evenly and place in the grinding chamber for grinding, nitrogen protection is circulated throughout the grinding process, and the grinding chamber is controlled The temperature in the body is controlled at 15-30°C, and the grinding time is between about 5-8 μm according to the particle size distribution D90 of the powder. A co-ground solid composition is obtained.
Embodiment 2
[0043] Embodiment 2, solid composition
[0044] Take celecoxib 100g, polyvinylpyrrolidone K 30 45g, poloxamer 40g, disodium hydrogen phosphate 1.0g and sodium hydroxide 0.5g (where polyvinylpyrrolidone K 30 and poloxamer as a dispersing co-solvent, disodium hydrogen phosphate and sodium hydroxide are basic compounds), mixed evenly and then placed in a ball mill for co-grinding, nitrogen protection is circulated throughout the grinding process, and the temperature in the chamber of the ball mill is controlled not to exceed At 30°C, the grinding time is between about 5 and 20 μm according to the particle size distribution D90 of the powder. A co-ground solid composition is obtained.
Embodiment 3
[0045] Embodiment 3, solid composition
[0046] Take celecoxib 100g, β-cyclodextrin 45g, hydroxypropyl cellulose 30g, disodium hydrogen phosphate 0.5g (wherein β-cyclodextrin and hydroxypropyl cellulose are dispersing cosolvents, disodium hydrogen phosphate is Alkaline compounds), mixed evenly and placed in a ball mill to grind together for 80 minutes. During the grinding process, nitrogen protection was circulated throughout the process, and the temperature in the chamber of the ball mill was controlled at 15-30°C. The grinding time was based on the particle size distribution of the powder D90 at about 5-20 μm between. A co-ground solid composition is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| D90 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com