Preparation methods and application of novel ortho-ester monomer and acid-sensitive polymeric gene carrier thereof
A technology of acid-sensitive polymer and gene carrier, which is applied in the direction of introducing foreign genetic material by using carrier, organic chemistry, recombinant DNA technology, etc. It can solve the problems of reducing the cycle time of the gene delivery system, reducing the efficiency of gene transfection, etc., and achieving high gene efficiency. The effects of transfection efficiency, low cytotoxicity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
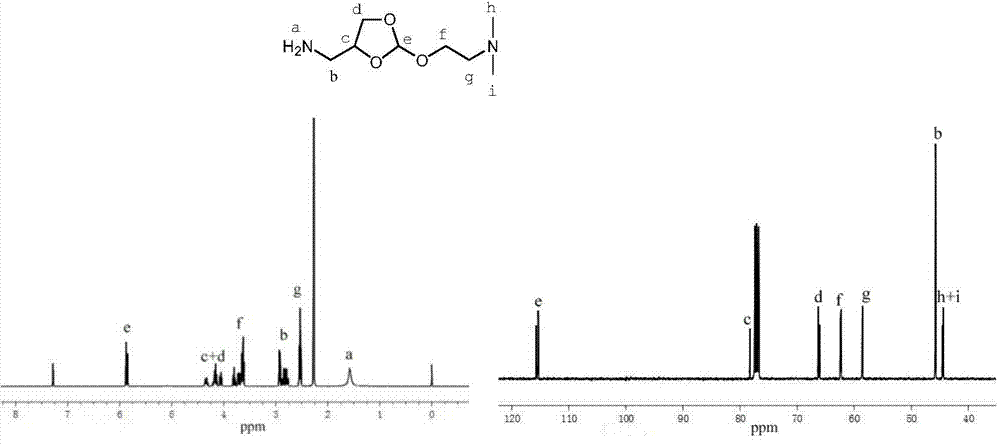
[0038] The synthesis method of 4-aminomethyl-2-(N,N-dimethylethoxy)-[1,3]dioxane monomer is realized through the following steps:
[0039] 1) 2,2,2-trifluoro-N-(2-(5'-N,N-dimethylethoxy)-[1,3]dioxane-4-methylene)acetamide preparation
[0040]Under nitrogen atmosphere, 11.66g (50.88mmol) 2,2,2-trifluoro-N-(2-methoxy-[1,3]dioxane-4-methylene)acetamide, 86.51mg( 0.32mmol) p-toluenesulfonic acid pyridinium salt and 13.95g (53.38mmol) 2-dimethylaminoethanol, heated to 130 ° C for 2 hours, cooled to room temperature, and obtained 25.63 g of the initial product of light yellow solid, no need for purification , which can be directly used in the next reaction.
[0041] 2) Preparation of 4-aminomethyl-2-(N,N-dimethylethoxy)-[1,3]dioxane
[0042] 25.63 g of 2,2,2-trifluoro-N-(2-(5'-N,N-dimethylethoxy)-[1,3]dioxane-4-methylene)ethane The crude product of the amide monomer was dissolved in 235ml of tetrahydrofuran, stirred and dissolved, and then 235ml of 2.0M sodium hydroxide solution...
Embodiment 2
[0044] The synthesis method of 4-aminomethyl-2-(N,N-dimethylethoxy)-[1,3]dioxane monomer is realized through the following steps:
[0045] 1) 2,2,2-trifluoro-N-(2-(5'-N,N-dimethylethoxy)-[1,3]dioxane-4-methylene)acetamide preparation
[0046] Under nitrogen atmosphere, 10.60g (46.26mmol) 2,2,2-trifluoro-N-(2-methoxy-[1,3]dioxane-4-methylene)acetamide, 78.66mg( 0.29mmol) pyridinium p-toluenesulfonate and 12.68g (48.54mmol) 2-dimethylaminoethanol, heated to 130°C for 2 hours, cooled to room temperature to obtain 23.29g of the initial product as a light yellow solid, no need for purification , which can be directly used in the next reaction.
[0047] 2) Preparation of 4-aminomethyl-2-(N,N-dimethylethoxy)-[1,3]dioxane
[0048] 23.29 g containing 2,2,2-trifluoro-N-(2-(5'-N,N-dimethylethoxy)-[1,3]dioxane-4-methylene)ethane The crude product of the amide monomer was dissolved in 213ml of tetrahydrofuran, stirred and dissolved and then added with 213ml of 2.0M sodium hydroxide sol...
Embodiment 3
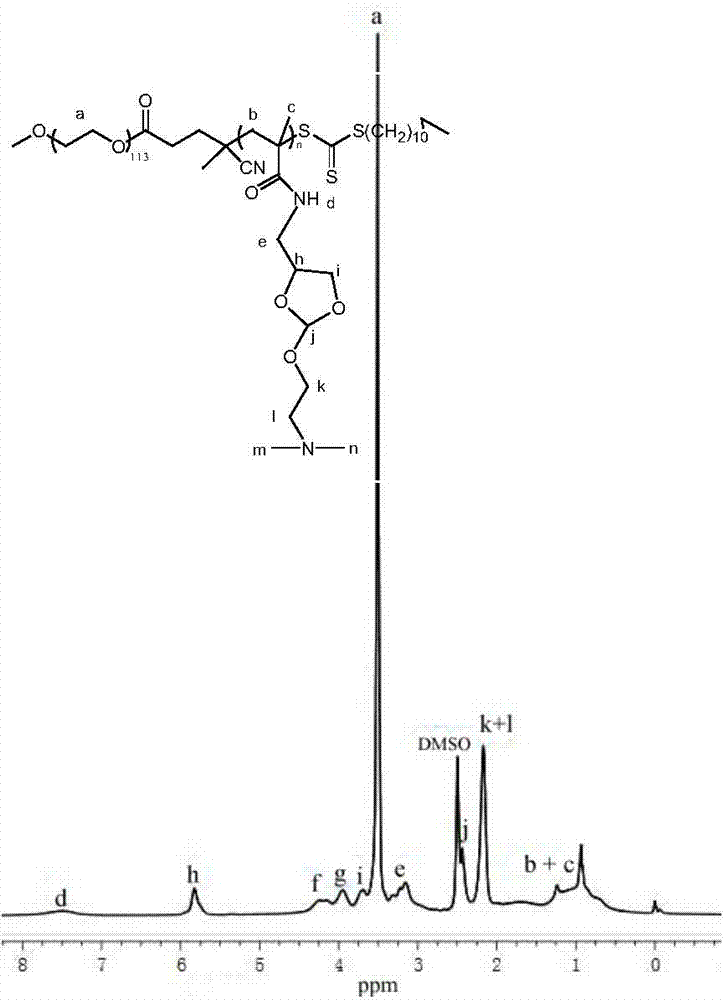
[0050] Accurately weigh 1.00g (5.46mmol) N-succinimide methacrylate, 0.328g (0.06mmol) macromolecular chain transfer agent, and 1.99mg (0.01mmol) initiator in a clean and dry glass reaction bottle , and finally add 2.5 ml of N,N-dimethylformamide from which water has been removed. Introduce nitrogen gas into the polymer, seal the tube by bubbling continuously for more than 30 minutes, and then put the reaction bottle into an oil bath at 70°C for polymerization. After reacting for 2 hours, dissolve the obtained primary product in N,N-dimethyl In formamide, after shaking and dissolving completely, the polymer was precipitated with a large amount of ether and filtered. The sedimentation was repeated twice, and finally the obtained polymer was vacuum-dried to constant weight. Under nitrogen protection, 0.3g (0.032mmol) of the obtained copolymer, 0.40g (2.10mmol) of the new orthoester monomer 4-aminomethyl-2-(N,N-dimethylethoxy)-[1 ,3] Dioxane was accurately weighed and put into ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com