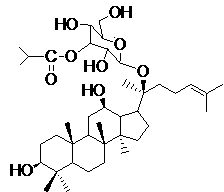
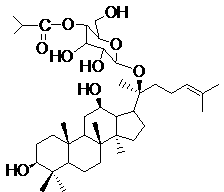
Synthetic method for improving ginsenoside M1 and iso-butyryl chloride mono-esterification selectivity
A technology of isobutyryl chloride monoester and ginsenoside, applied in the production of steroids, organic chemistry, bulk chemicals, etc., can solve the problems of difficult separation and purification, limited source of EM1, poor water solubility of series M1 derivatives, etc. The effect of separation and purification, optimization of selectivity, and improvement of yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Synthesis of monoetherification of ginsenoside M1 with tert-butyldimethylsilyl chloride
[0030] Add 100 mg of ginsenoside M1 and 5 times the equivalent of tert-butyldimethylsilyl chloride to a 25 mL Shouliang bottle equipped with an electromagnetic stirrer, under nitrogen protection, add an appropriate amount of triethylamine to dissolve in an ice-water bath, and react for 10 After 1 min, it was raised to room temperature, and the reaction progress was monitored by thin-layer chromatography. After 24 h of reaction, saturated ammonium chloride solution was added to stop the reaction. via CH 2 Cl 2 (3×10 mL) extraction, Brine washed the organic phase, anhydrous Na 2 SO 4 Dry and concentrate the solvent. Column chromatography purification
[0031] Procedure: amount of silica gel, 5 g; mobile phase, methanol / dichloromethane;
[0032] After gradient elution, the Rf value was 9 / 34 (8%MeOH / CH 2 Cl 2 ) of the product 100 mg
[0033] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com