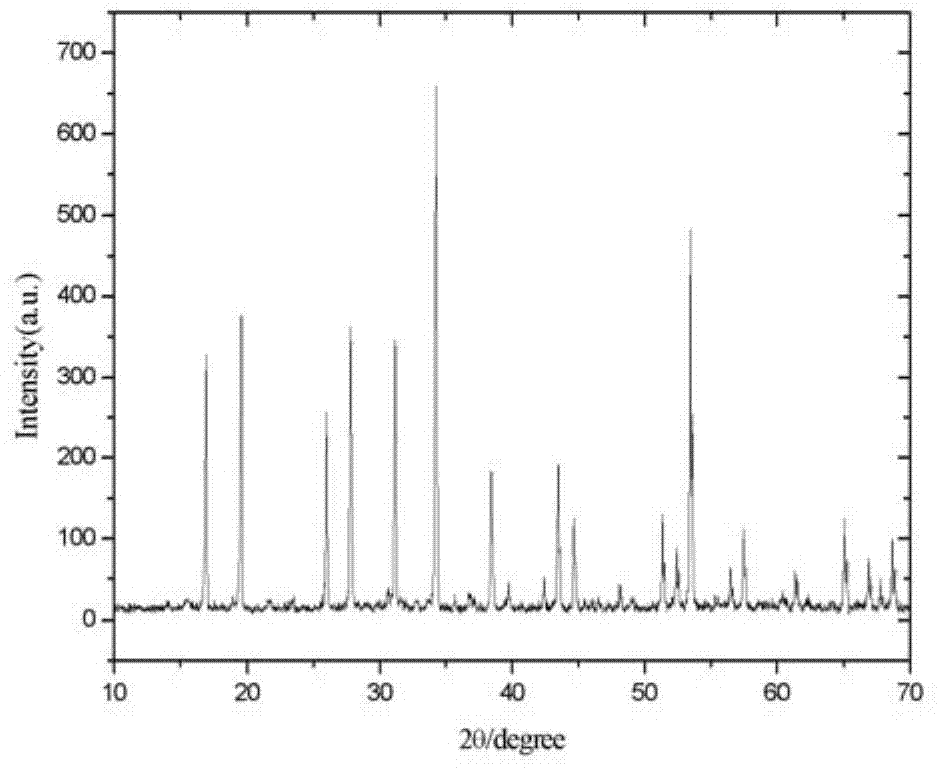
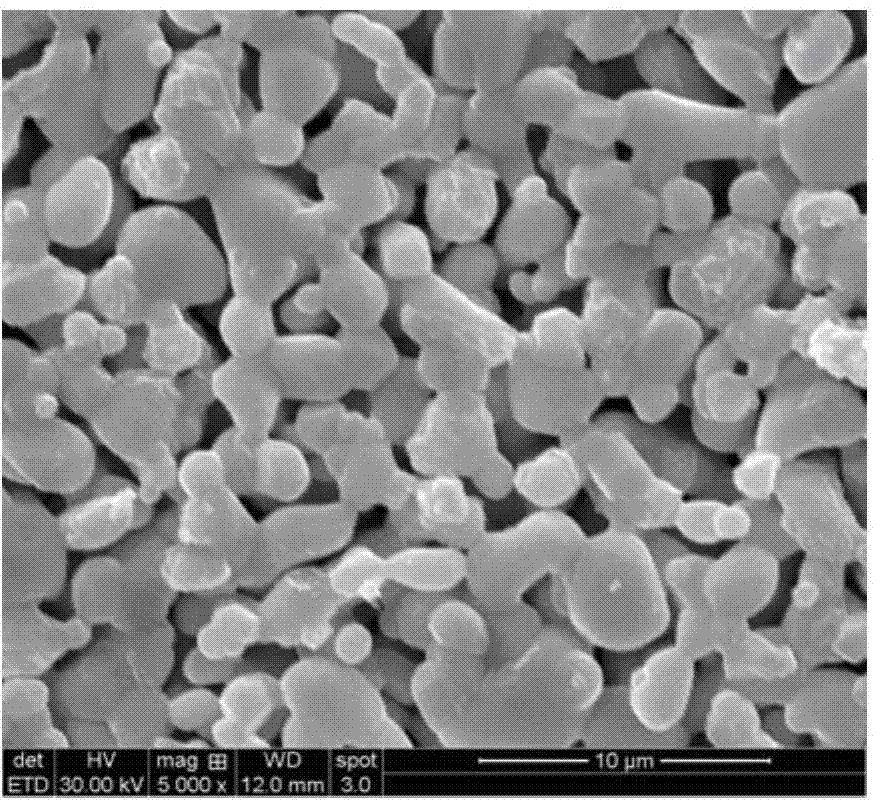
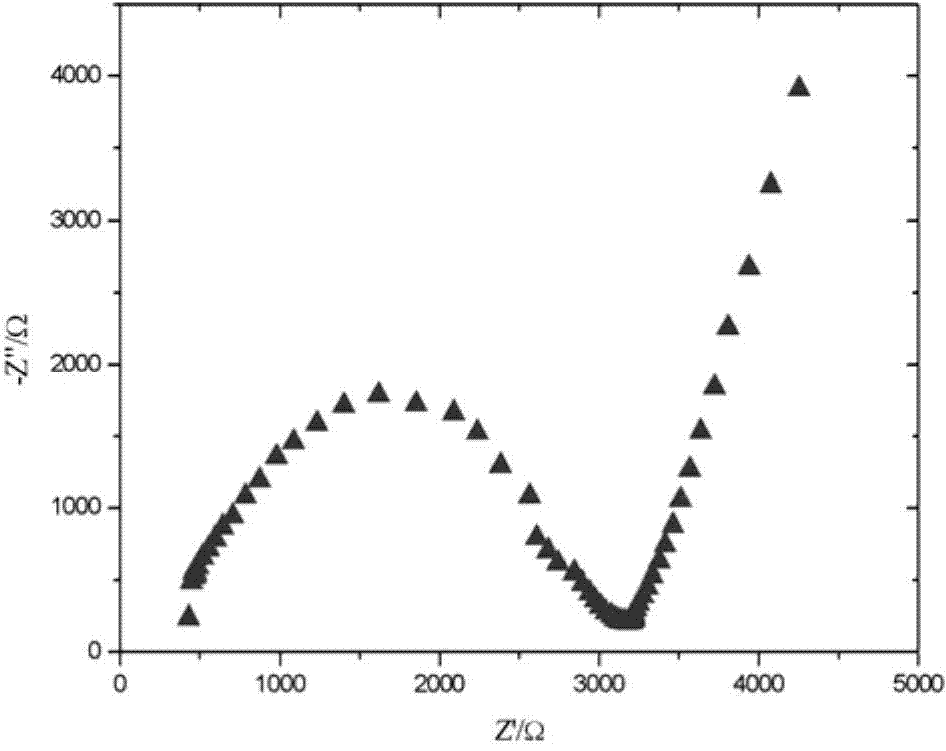
Garnet-structure lithium lanthanum tantalate-based solid electrolyte material and preparation method thereof
A technology based on lanthanum lithium tantalate and solid electrolyte, which is applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc. It can solve the problems of harsh preparation conditions, poor electrochemical stability, and low conductivity, and achieve mild calcination conditions , stable lithium ion content and short calcination time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The concrete steps of preparation are:
[0037] Step 1, follow Li 5+δ La 3-x A x Ta 2-y B y o 12 The composition ratio, where x=0, y=1.0, first weigh the corresponding amount of tantalum pentoxide, add to the 2 C 2 o 4 If it is insoluble, it can be heated to make it clear, and then add nitrate or acetate of lithium, lanthanum, and tantalum doping compound (B=Nb) to the solution to prepare a solution, and finally add it to the mixed solution Add EDTA, wherein the molar ratio of EDTA to metal ions in the mixed solution is 0.08, place it at 80° C. and stir for 1 h to obtain a transparent and clear sol.
[0038] Step 2, after adding a water-soluble polymer to the sol, place it under stirring at 50°C to form a gel, wherein the mass ratio of the water-soluble polymer to EDTA is 2:30, and the water-soluble polymer The polymer is polyethylene glycol.
[0039]Step 3, firstly dry the gel at 80°C for 24 hours to obtain a fluffy xerogel, then calcinate the xerogel at 700°...
Embodiment 2
[0041] The concrete steps of preparation are:
[0042] Step 1, follow Li 5+δ La 3-x A x Ta 2-y B y o 12 The composition ratio, where x=0.25, y=0.75, first weigh the corresponding amount of tantalum pentoxide, add to the 2 C 2 o 4 If it is insoluble, it can be heated to make it clear, and then add nitrate or acetic acid of lithium, lanthanum, lanthanum doping compound (A=K) and tantalum doping compound (B=Nb) to the solution After the salt was formulated into a solution, EDTA was finally added to the mixed solution, wherein the molar ratio of the added EDTA to the metal ion in the mixed solution was 0.08, and it was placed at 50° C. and stirred for 1.5 h to obtain a transparent and clear sol.
[0043] Step 2, after adding a water-soluble polymer to the sol, place it under stirring at 60°C to form a gel, wherein the mass ratio of the water-soluble polymer to EDTA is 1.5:20, and the water-soluble polymer The polymer is polyethylene glycol.
[0044] Step 3, firstly dry...
Embodiment 3
[0046] The concrete steps of preparation are:
[0047] Step 1, follow Li 5+δ La 3-x A x Ta 2-y B y o 12 The composition ratio, where x=0.25, y=0.25, first weigh the corresponding amount of tantalum pentoxide, add to the 2 C 2 o 4 If it is insoluble, it can be heated to make it clear, and then add the nitrate or acetic acid of lithium, lanthanum, lanthanum doping compound (A=K) and tantalum doping compound (B=In) to the solution After the salt was formulated into a solution, EDTA was finally added to the mixed solution, wherein the molar ratio of the added EDTA to the metal ion in the mixed solution was 0.06, and it was placed at 50° C. and stirred for 1.2 h to obtain a transparent and clear sol.
[0048] Step 2, after adding a water-soluble polymer to the sol, place it under stirring at 70°C to form a gel, wherein the mass ratio of the water-soluble polymer to EDTA is 1.5:15, and the water-soluble polymer The polymer is polyethylene glycol.
[0049] Step 3, firstly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| crystal size | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com