Preparation method of capecitabine
A technology of capecitabine and acetyl, which is applied in the field of drug synthesis, can solve problems such as difficult dissolution, lower product yield, and high cost, and achieve the effects of improving yield and purity, increasing product yield, and saving reaction costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
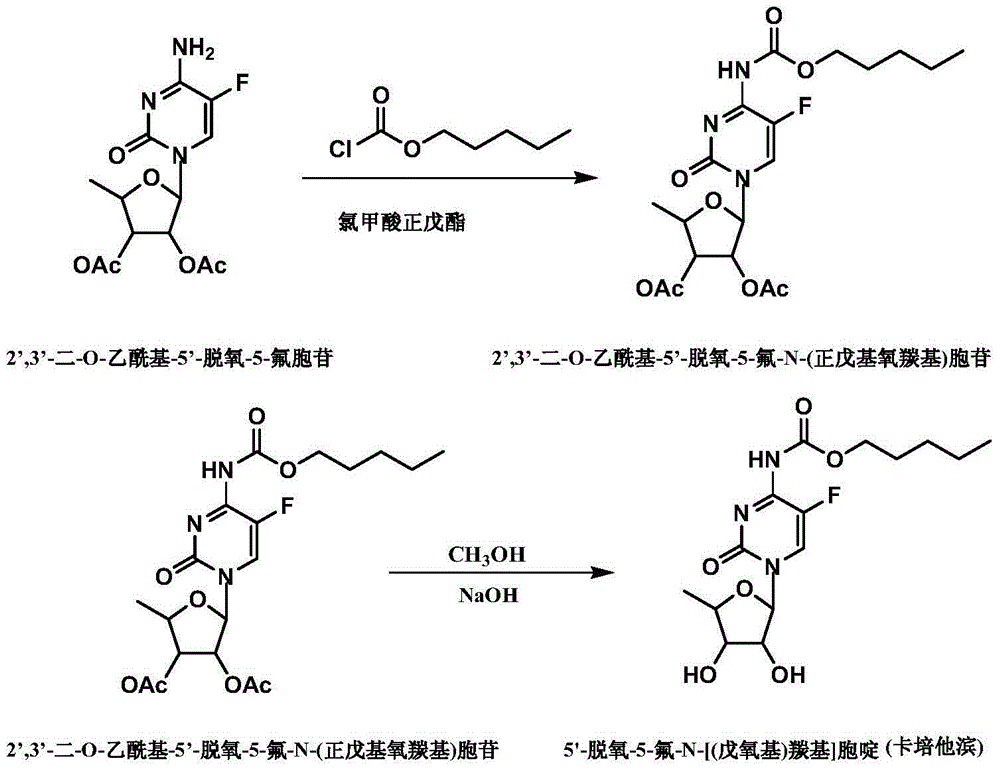
[0029] Example 12', Preparation of 3'-di-O-acetyl-5'-deoxy-5-fluoro-N-(n-pentyloxycarbonyl)cytidine
[0030] 20g of 2’,3’-di-O-acetyl-5’-deoxy-5-fluorocytidine was dissolved in 50ml of dichloromethane at room temperature, added 4ml of pyridine, and stirred until dissolved. 10 ml of n-pentyl chloroformate was added dropwise at -5°C to -10°C. After the dropwise addition was completed, the reaction was carried out for 2 hours. Wash with 60 ml of water for 15 minutes, twice, and discard the aqueous layer to obtain an organic layer. Concentrate the organic layer under reduced pressure until no distillate flows out, add 80ml of isopropyl ether and 4ml of acetonitrile into the oily substance, dissolve them all at room temperature, slowly cool down to 0°C-5°C, and slowly crystallize to obtain white powder 2',3' -Di-O-acetyl-5'-deoxy-5-fluoro-N-(n-pentyloxycarbonyl)cytidine 17g, mass yield 85%, purity 99.86%.
Embodiment 2
[0031] The preparation of embodiment 2 capecitabine crude product
[0032] Add 10g of 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N-(n-pentyloxycarbonyl)cytidine to 27ml of methanol at room temperature until dissolved, and cool down to -7°C Add 15 ml of 5N sodium hydroxide solution dropwise, and react at -3°C for 0.5 hours after the dropwise addition. After the reaction is completed, add concentrated hydrochloric acid to adjust the pH to 4.5-4.6. After the dropwise addition, add 50ml of dichloromethane to extract for 15 minutes, extract twice, combine the organic layers, add 30ml of water to wash for 15 minutes, add anhydrous sodium sulfate to the organic layer Dry, filter, concentrate under reduced pressure until viscous, slowly add 150ml of toluene dropwise for crystallization for 2 hours, and obtain 8.7g of crude capecitabine. Suction filtration, washing with toluene. The mass yield is 87%, and the purity is 99.86%.
Embodiment 3
[0033] Embodiment 3 capecitabine crude product refining
[0034] 3g of capecitabine crude product was dissolved in 10ml of dichloromethane, dissolved at room temperature until clarified, 50ml of toluene was added dropwise, and after the dropping, crystallized for 2 hours. Suction filtration and washing with toluene yielded 2.79 g of capecitabine product. The mass yield is 93%, and the purity is 99.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com