A pure-phase nickel ferrite nanomaterial and its preparation method, and a preparation method of aromatic amine
A nanomaterial, nickel ferrite technology, applied in the field of nickel ferrite and its preparation method, and aromatic amine preparation field, can solve the problems of high price of precious metals, increase production cost, difficult to meet industrialization process, etc., and achieve high catalytic activity and efficiency High and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
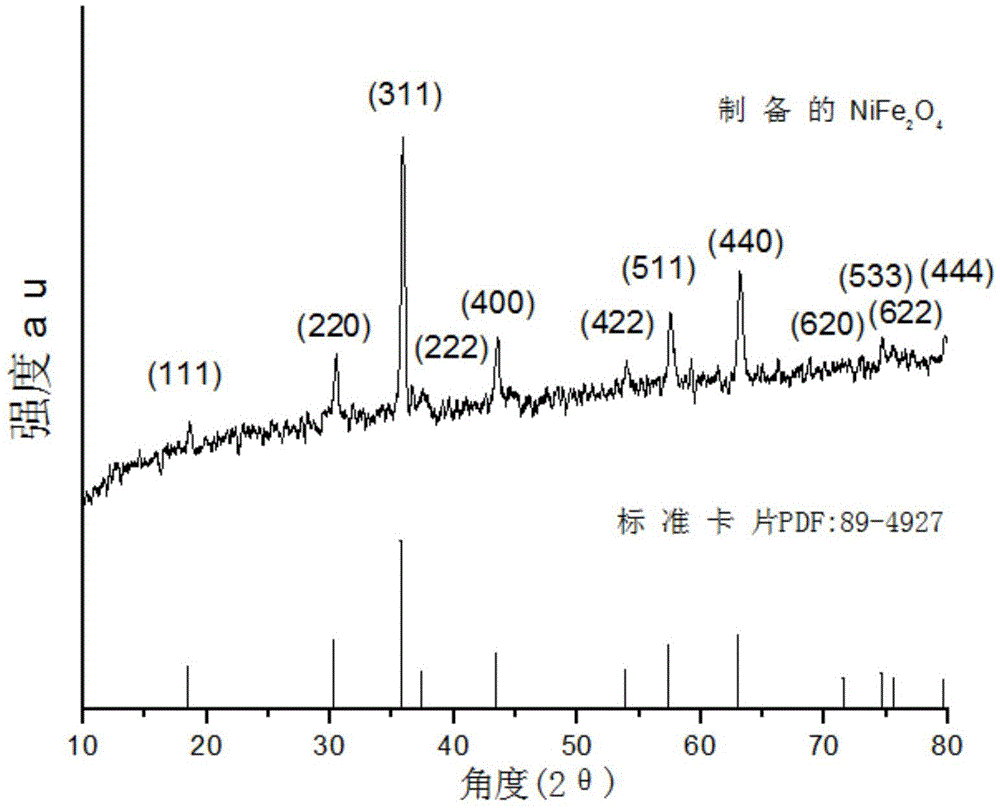
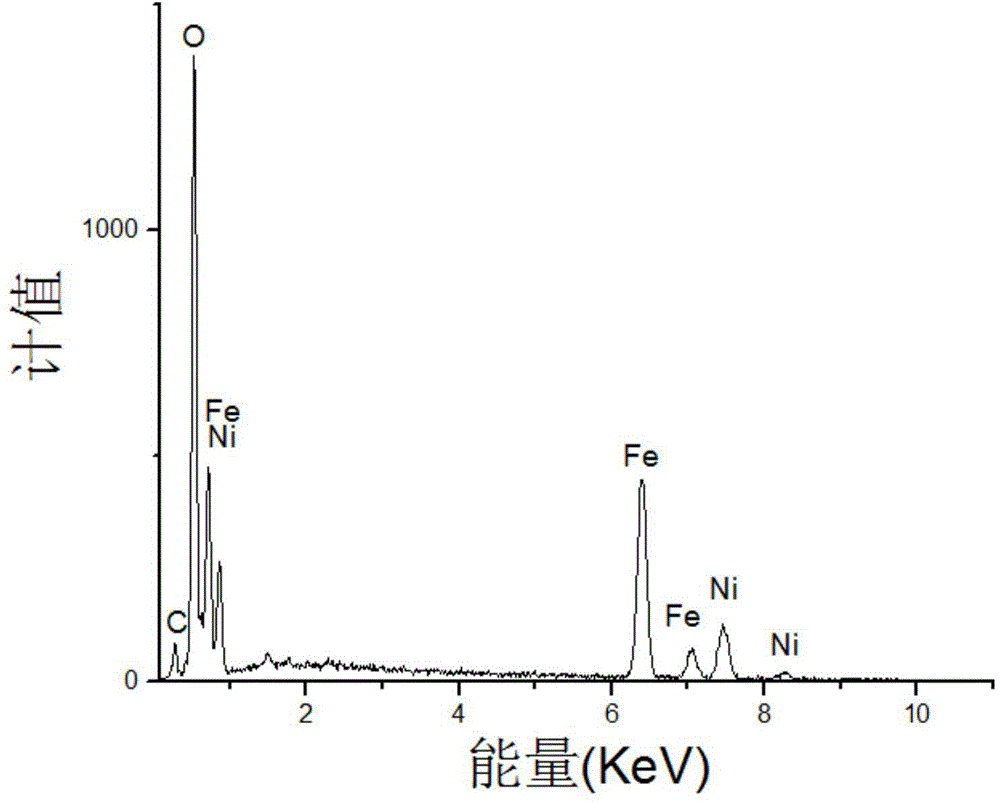
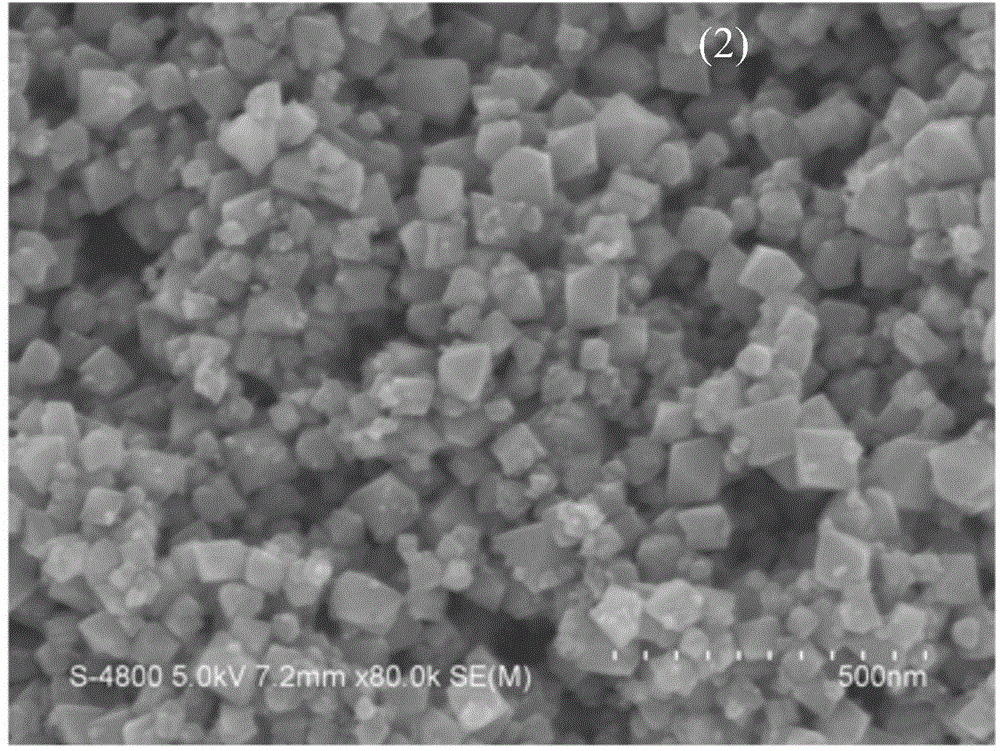
[0052] Fig. 3(a) is a scanning electron micrograph of the pure-phase nickel ferrite prepared in Example 1, which is octahedral nano-magnetic particles with a particle size of 70nm.
[0053] The preparation method of pure phase nickel ferrite nanometer material comprises the following steps:
[0054] a. Stir and mix 1mmol of cetyltrimethylammonium bromide with 5mL of n-hexane and 1.7mL of isopropanol to obtain solution A; mix 1.5mmol of cetyltrimethylammonium bromide with 5mL of n-hexane and 2.5mL of isopropanol were stirred and mixed to obtain solution B;
[0055] b. Take 0.7mL of nickel chloride hexahydrate and ferric nitrate nonahydrate mixed salt solution, add it dropwise into the solution A, stir for 20 minutes to obtain suspension A, put the mixed salt solution in 0.7mL Contains 0.125 mmol of nickel chloride hexahydrate and 0.25 mmol of ferric nitrate nonahydrate; add 6 mL of 0.5 mol / L potassium hydroxide solution dropwise to the solution B, and stir for 20 minutes to ob...
Embodiment 2
[0060] Fig. 3(b) is a scanning electron micrograph of the pure-phase nickel ferrite prepared in Example 2, which is octahedral nano-magnetic particles with a particle size of 80 nm.
[0061] The preparation method of pure phase nickel ferrite nanometer material comprises the following steps:
[0062] a. Stir and mix 25mL of 5mmol cetyltrimethylammonium bromide n-hexane and 9.2mL of isopropanol to obtain solution A; mix 6mmol cetyltrimethylammonium bromide with 30mL of n-hexane and 11mL of isopropanol was stirred and mixed to obtain solution B;
[0063] b. Take 2mL of mixed salt solution of nickel chloride hexahydrate and ferric nitrate nonahydrate, add it dropwise to the solution A, and stir for 30 minutes to obtain suspension A. The 2mL mixed salt solution contains nickel chloride hexahydrate 0.376mmol and 0.752mmol of ferric nitrate nonahydrate; 10mL of 1mol / L sodium hydroxide solution was added dropwise to the solution B, and stirred for 30 minutes to obtain a suspension B...
Embodiment 3
[0068] Fig. 3(c) is a scanning electron micrograph of the pure-phase nickel ferrite prepared in Example 3, which is octahedral nano-magnetic particles with a particle size of 100 nm.
[0069] The preparation method of pure phase nickel ferrite nanometer material comprises the following steps:
[0070] a. Stir and mix 1.5mmol of cetyltrimethylammonium bromide with 17mL of n-hexane and 6.1mL of isopropanol to obtain solution A; mix 8mmol of cetyltrimethylammonium bromide with 20mL of n-hexane Stir and mix with 5.5mL of isopropanol to obtain solution B;
[0071] b. Take 1.2mL of nickel sulfate hexahydrate and ferric chloride hexahydrate mixed salt solution, dropwise added to the solution A, stirred for 40 minutes, to obtain suspension A, the mixed salt solution contained six 0.225 mmol of nickel sulfate hydrate and 0.45 mol of ferric chloride hexahydrate; 12 mL of 0.25 mol / L sodium hydroxide solution was added dropwise to the solution B, and stirred for 40 minutes to obtain susp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com