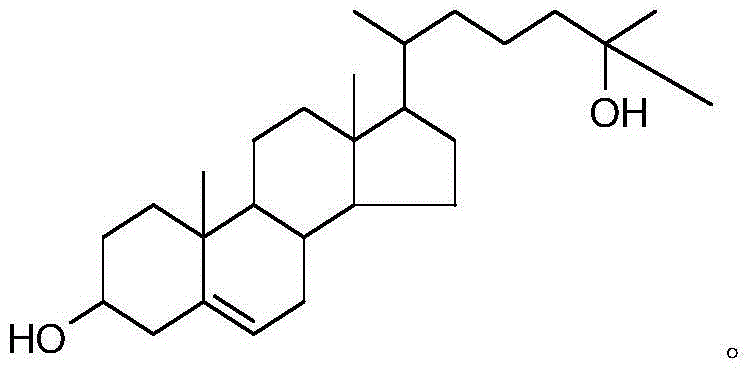
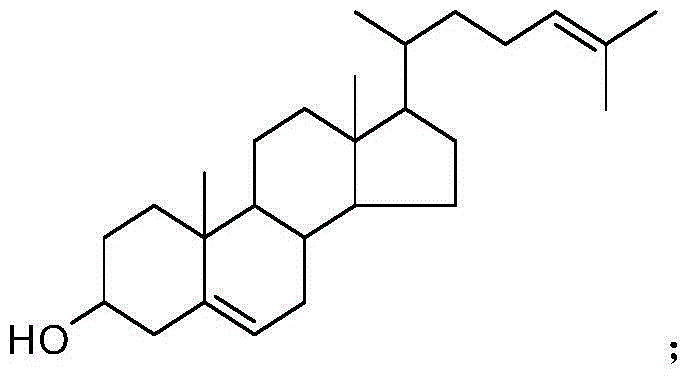
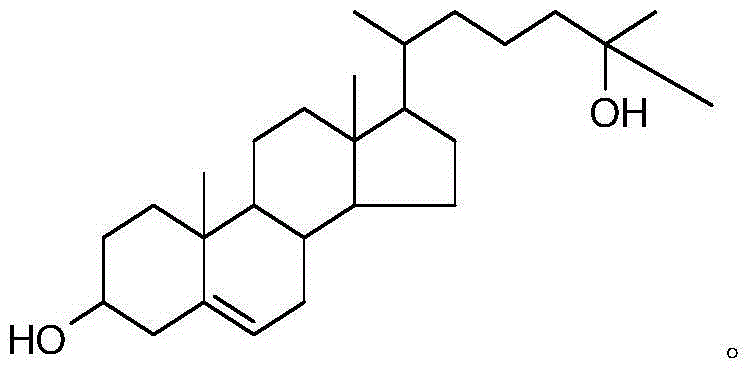
The synthetic method of 25-hydroxycholesterol
A technology of hydroxycholesterol and synthesis method, applied in the direction of steroids, organic chemistry, etc., can solve the problems of poor selectivity and low yield, and achieve the effects of high selectivity, lower reaction temperature, and higher yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, a kind of synthetic method of 25-hydroxycholesterol, carries out following steps successively:
[0047] 1) With 120mL pyridine as solvent and 30mL pyridine (about 0.372mol) as acid-binding agent, add 20g of 24-dehydrocholesterol (about 0.052mol) and 0.2g of DMAP, and add 10g (about 0.097mmol) dropwise at room temperature ) acetic anhydride (dropped in about 30 minutes). The reaction process was monitored by TLC. After the dropwise addition, the reaction was continued at room temperature for 10 h, and the reaction ended.
[0048] The reaction solution was washed with water (the amount of water used was 50ml×2), pickled (the amount used was 50ml×2 with dilute hydrochloric acid solution with a volume concentration of 5%), and extracted with dichloromethane (the amount used was 30ml×3). Wash with saturated sodium bicarbonate solution until neutral, dry over anhydrous sodium sulfate (about 5 g) and remove the solvent (ie, dichloromethane) to obtain 19.1 g of a...
Embodiment 2
[0056] Embodiment 2, a kind of synthetic method of 25-hydroxycholesterol,
[0057] In this embodiment 2, steps 1) and 2) are different from embodiment 1, the difference lies in:
[0058] 1) With 100mL of dichloromethane as a solvent, add 20g of 24-dehydrocholesterol (about 0.052mol), 0.2g of DMAP, 10.1g (about 0.1mmol) of triethylamine, and drop 10g (about 0.097mmol) of vinegar at room temperature anhydride. The reaction process was monitored by TLC. After the dropwise addition, the reaction was continued at room temperature for 10 h, and the reaction ended.
[0059] The reaction solution was washed successively with dilute hydrochloric acid (volume concentration 5%, dosage 50ml×2), saturated sodium bicarbonate solution (30ml×2), saturated sodium chloride solution (30ml) and dried with anhydrous sodium sulfate (about 5g) , and reduced pressure (0.1 MPa) to remove the solvent (ie dichloromethane) by rotary evaporation to obtain 19.0 g of acetylated 24-dehydrocholesterol with ...
Embodiment 3
[0062] Embodiment 3, a kind of synthetic method of 25-hydroxycholesterol,
[0063] In this embodiment 3, steps 1), 2) are different from embodiment 1, the difference lies in:
[0064] 1) With 100mL of dichloromethane as a solvent, add 20g of 24-dehydrocholesterol (about 0.052mol), 0.2g of DMAP, 10.1g (about 0.1mmol) of triethylamine, and drop 14.1g (about 0.1mol) of triethylamine at room temperature ) Benzoyl chloride, after the dropwise addition, the temperature was raised to reflux, and the reaction was terminated after 24 hours of reaction.
[0065] The rest is basically the same as step 1) of Example 2 to obtain 23.2 g of benzoylated 24-dehydrocholesterol with a yield of 95.0%.
[0066] 2) Use 0.4g (2mmol) of 40% peracetic acid as the epoxidation reagent to replace the "38% (mass concentration) peracetic acid 0.4g (2mmol)" in Example 1, and change the reaction temperature from -10°C to -20°C.
[0067] The rest are basically the same as step 2 of Example 1); 0.27g of epo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com