Preparation method and application of CO<2+>-doped titanium dioxide self-cleaning film
A titanium dioxide, self-cleaning technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, chemical/physical processes, etc., can solve the problem of poor film formation and repeatability, and degradation The ability has not been significantly improved, the process technology is complicated, etc., to achieve the effect of reducing compounding, convenient operation, and simple process preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
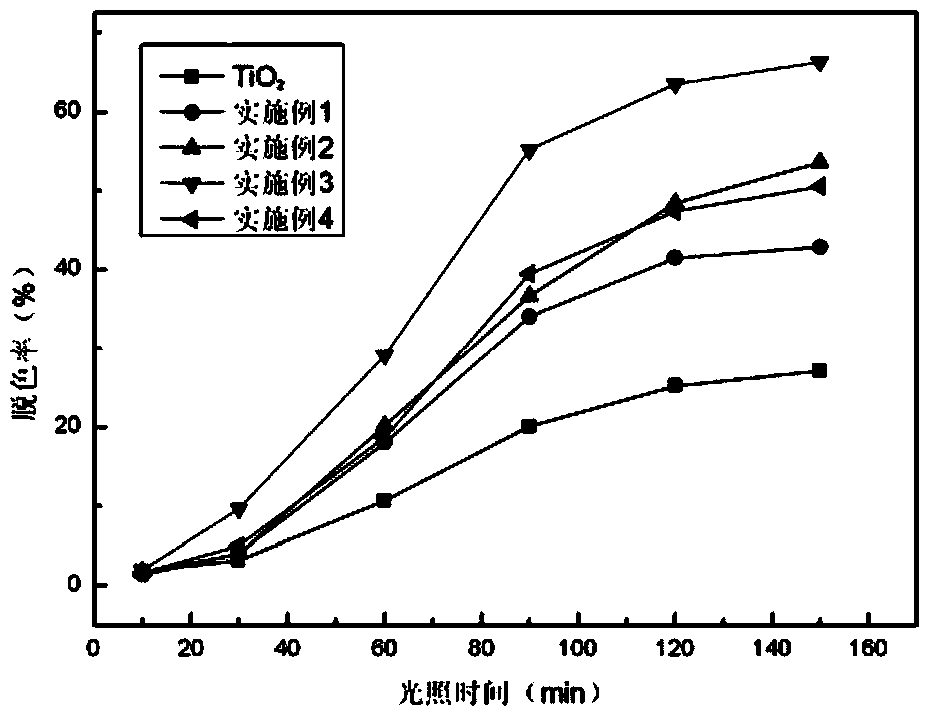
Image
Examples
Embodiment 1
[0025] 1) The analytically pure butyl phthalate (C 16 h 36 o 4 Ti) and analytically pure ethanol (C 2 h 5 OH) according to the volume ratio of 1:0.5 and mix evenly, then add analytically pure diethanolamine (C 4 h 11 NO 2 ), to obtain a transparent solution A; wherein, the volume content of diethanolamine in the transparent solution A is 3%;
[0026] 2) Deionized water, analytically pure acetylacetone (C 5 h 8 o 2 ) and analytically pure absolute ethanol were uniformly mixed at a volume ratio of 1:1:10 to obtain a transparent solution B;
[0027] 3) Slowly add transparent solution B into transparent solution A and keep stirring evenly to obtain a mixed solution; then add analytically pure cobalt acetate tetrahydrate (Co(CH 3 COO) 2 4H 2 O), and use analytically pure hydrochloric acid to adjust the pH value of the mixed solution to 2.0, and form a uniform sol after magnetic stirring for 5 hours; the uniform sol is aged at room temperature for 10 hours to obtain the ...
Embodiment 2
[0031] 1) The analytically pure butyl phthalate (C 16 h 36 o 4 Ti) and analytically pure ethanol (C 2 h 5 OH) according to the volume ratio of 1:1, and then add analytically pure diethanolamine (C 4 h 11 NO 2 ), to obtain a transparent solution A; wherein, the volume content of diethanolamine in the transparent solution A is 5%;
[0032] 2) Deionized water, analytically pure acetylacetone (C 5 h 8 o 2 ) and analytically pure absolute ethanol were mixed uniformly at a volume ratio of 1:2:15 to obtain a transparent solution B;
[0033] 3) Slowly add transparent solution B into transparent solution A and keep stirring evenly to obtain a mixed solution; then add analytically pure cobalt acetate tetrahydrate (Co(CH 3 COO) 2 4H 2 O), and use analytically pure hydrochloric acid to adjust the pH value of the mixed solution to 5.0, and form a homogeneous sol after magnetic stirring for 1 h; the homogeneous sol is aged at room temperature for 10 h to obtain the gel C require...
Embodiment 3
[0037] 1) The analytically pure butyl phthalate (C16 h 36 o 4 Ti) and analytically pure ethanol (C 2 h 5 OH) according to the volume ratio of 1:3 and mix evenly, then add analytically pure diethanolamine (C 4 h 11 NO 2 ), to obtain a transparent solution A; wherein, the volume content of diethanolamine in the transparent solution A is 6%;
[0038] 2) Deionized water, analytically pure acetylacetone (C 5 h 8 o 2 ) and analytically pure absolute ethanol were mixed uniformly at a volume ratio of 1:1:20 to obtain a transparent solution B;
[0039] 3) Slowly add transparent solution B into transparent solution A and keep stirring evenly to obtain a mixed solution; then add analytically pure cobalt acetate tetrahydrate (Co(CH 3 COO) 2 4H 2 O), and use analytically pure hydrochloric acid to adjust the pH value of the mixed solution to 4.0, and form a uniform sol after magnetic stirring for 5 hours; the uniform sol is aged at room temperature for 48 hours to obtain the gel ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com