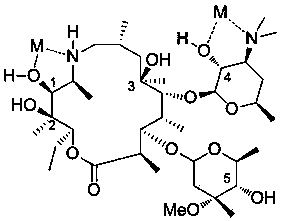
Synthetic method of tulathromycin
A technology of tyramectin and a synthesis method, which is applied in the field of tyramectin synthesis, can solve the problems of danger, high price, long reaction time and the like, and achieves the effects of safe operation, low cost and energy saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] S1. Dissolve 1.3g of N-chlorosuccinimide in 10ml of toluene, cool to 0°C, add dropwise a toluene solution containing 0.6g of dimethyl sulfide to obtain solution 1, and cool solution 1 to -25°C for later use. Dissolve 7.3g of azithromycin A in 25ml of toluene, add 0.5g of lithium hydroxide, stir at 25°C for 1 hour, then add dropwise to the above-mentioned mixed solution of N-chlorosuccinimide and dimethyl sulfide, keep React at -25°C for 2 hours, add dropwise a toluene solution containing 1g of triethylamine, return to room temperature after the dropwise addition, add dilute acid aqueous solution and stir, separate the organic phase, dry, filter, and recover the solvent to obtain 3.6g of ketone compound I , yield 50%.
[0030] S2. Take 1.13g of trimethylsulfonium chloride, add 20ml of dichloromethane, add 0.56g of potassium hydroxide, stir for half an hour to obtain solution 2, add dropwise to solution 2 containing 7.33g of ketone compound I dichloromethane solution, st...
Embodiment 2
[0033] S1. Dissolve 2.6g of N-chlorosuccinimide in 20ml of toluene, cool to 0°C, add dropwise a toluene solution containing 2.5g of dimethyl sulfide to obtain solution 1, and cool solution 1 to -25°C for later use. Dissolve 7.3g of azithromycin A in 25ml of toluene, add 3.2g of lithium trifluoromethanesulfonate, stir at 25°C for 1 hour, then add dropwise to the reaction mixture of N-chlorosuccinimide and dimethyl sulfide , kept at -25°C for 2 hours, added dropwise a toluene solution containing 2g of triethylamine, returned to room temperature after the dropwise addition, added dilute acid aqueous solution and stirred, separated the organic phase, dried, filtered, and recovered the solvent to obtain 6.2g of ketone compound I , 85% yield.
[0034] S2. Take 3.14g of trimethylsulfonium bromide, add 25ml of dichloroethane, add 2.4g of sodium hydroxide, stir for half an hour to obtain solution 2, add dropwise to solution 2 containing 7.33g of ketone compound I dichloroethane solut...
Embodiment 3
[0037] S1. Dissolve 1.6g of N-chlorosuccinimide in 12ml of dichloromethane, cool to 0°C, add dropwise a solution of dichloromethane containing 0.9g of dimethyl sulfide to obtain solution 1, and cool solution 1 to -25 ℃ for later use. Dissolve 7.3g of azithromycin A in 25ml of dichloromethane, add 2.2g of calcium chloride, stir at 25°C for 1 hour, then add dropwise to the reaction mixture of N-chlorosuccinimide and dimethyl sulfide , kept at -25°C for 2 hours, added dropwise a toluene solution containing 1.2g of triethylamine, returned to room temperature after the dropwise addition, added dilute acid aqueous solution and stirred, separated the organic phase, dried, filtered, and recovered the solvent to obtain 5.2g of ketone compound I , yield 72%.
[0038] S2. Take 2.41g trimethylsulfonium iodide, add 25ml acetonitrile, add 0.6g 60% sodium hydride, stir for half an hour, dropwise add 7.33g ketone compound I Acetonitrile solution was stirred at 80°C for 1 hour. Obtain 7.23...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com