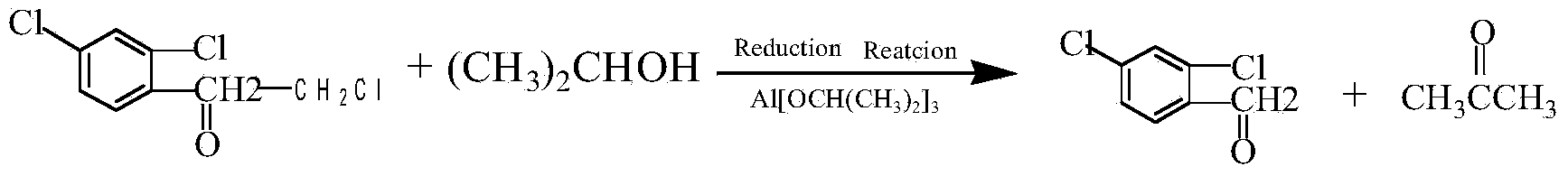Clean preparation method for 2,4-dichloro-alpha-chloromethyl benzyl alcohol
A chloromethyl benzyl alcohol, a clean technology, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of equipment corrosion, large pollution, high cost, and achieve mild reaction conditions and no environmental pollution. , the effect of convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The solutions and effects of the present invention will be further illustrated below in conjunction with examples of implementation.
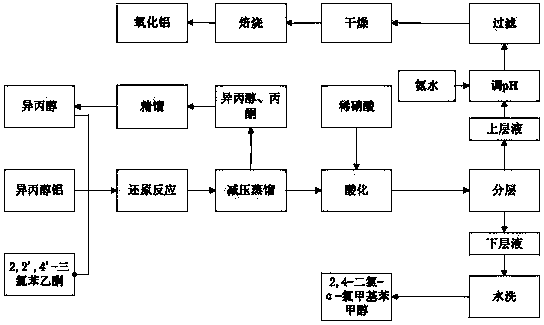
[0022] Add 120g of isopropanol, 14g of aluminum isopropoxide and 30g of 2,2',4'-trichloroacetophenone into a three-necked flask equipped with electric stirring, a thermometer, and a reflux condenser, and heat the reaction at 65°C for 5h; Control at 400-500r / min; Distill and remove isopropanol and acetone at a vacuum degree of -0.08-0.1mpa and a temperature below 70°C; add 15% dilute nitric acid, stir to neutralize aluminum isopropoxide, and remove the acidic water layer The feed liquid is washed with hot water at 55-65°C until neutral; the feed liquid is cooled and solidified to obtain 2,4-dichloro-α-chloromethyl benzyl alcohol with a melting point of 49.6-50.4°C; The mixture of propanol and acetone is rectified, and the fraction at 80-84°C is collected for repeated use. The acid water is adjusted to pH=8~9 with 25% ammonia water, and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com