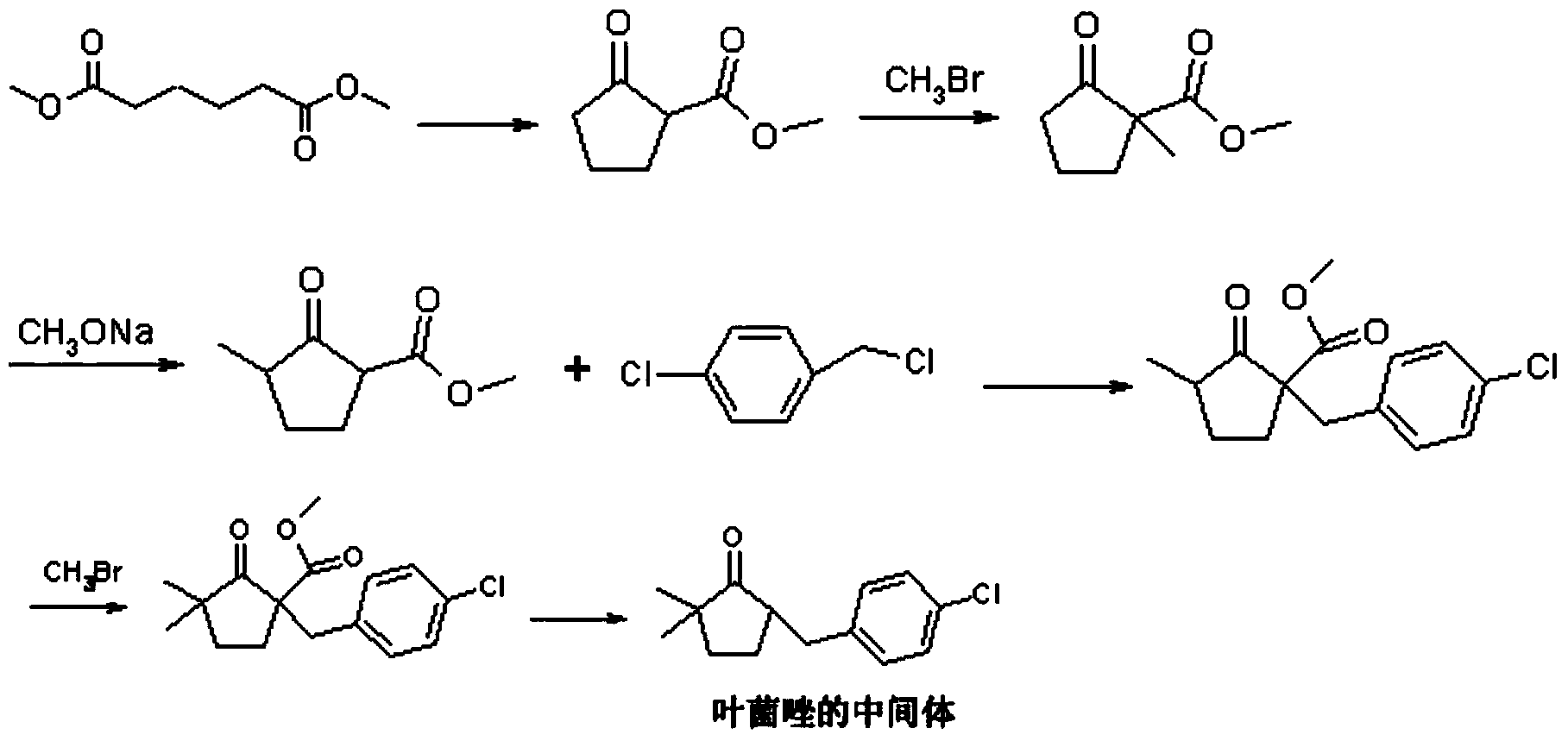
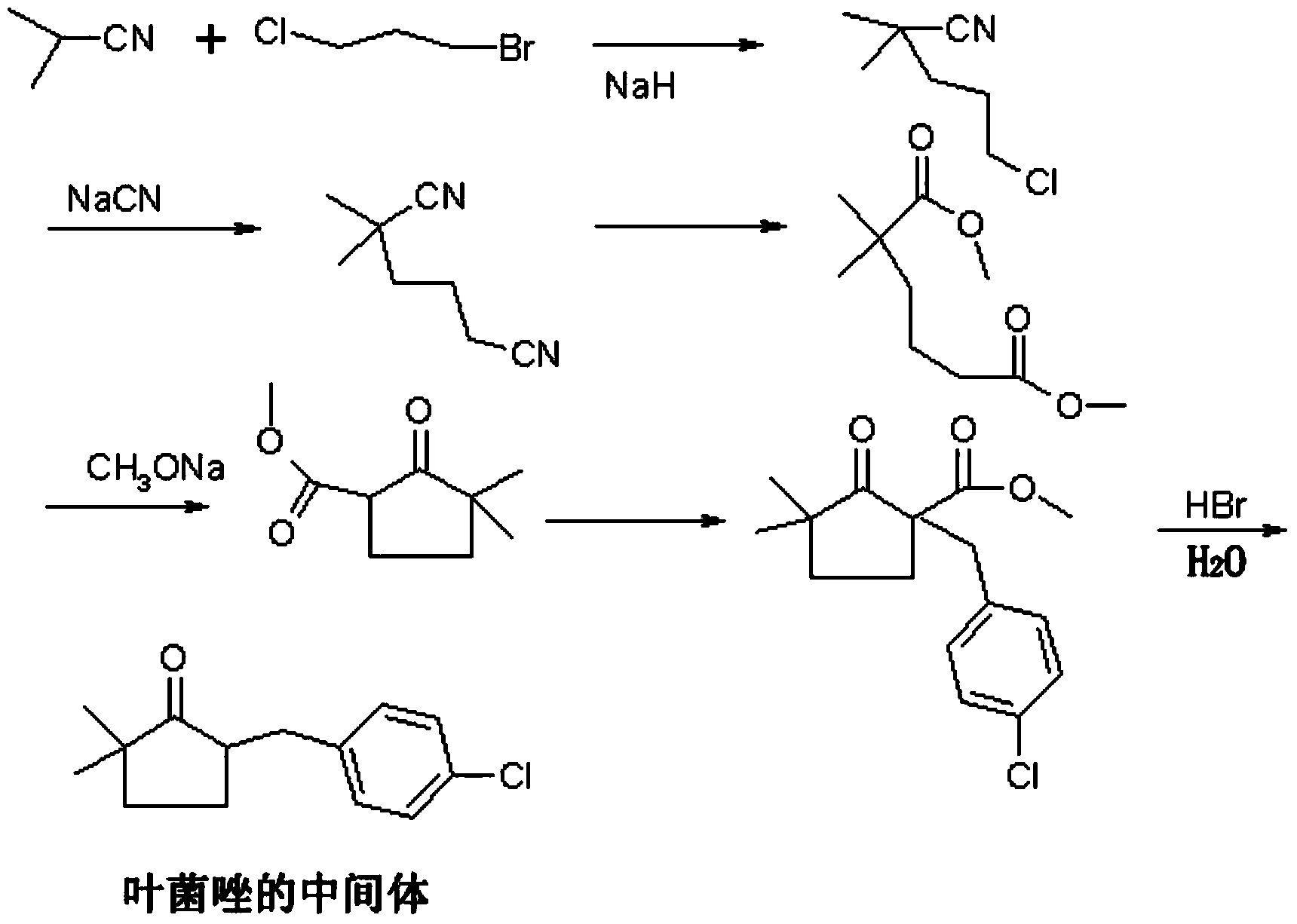
Preparation method of metconazole and intermediate thereof
A technology for metconazole and intermediates, which is applied in the field of preparation of metconazole and intermediates thereof, can solve the problems of low yield, many impurities, high price and the like, and achieves the effects of easy availability of raw materials, simple process and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The present embodiment provides a kind of preparation method of metconazole, concrete implementation is as follows:
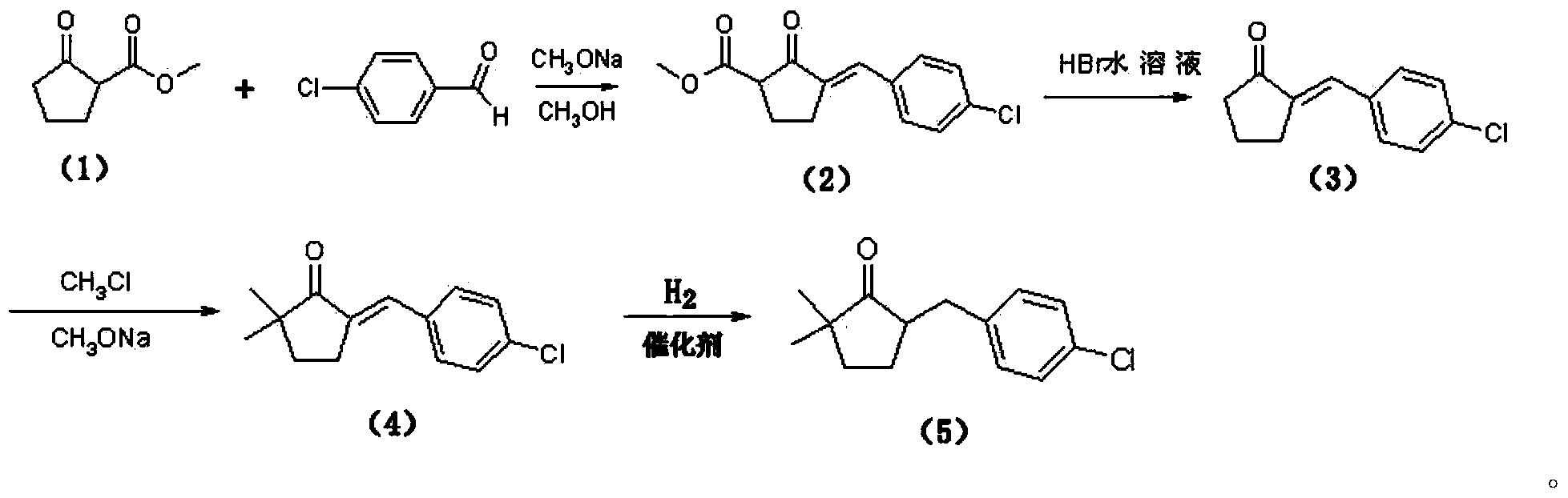
[0038] (a) Preparation of formula (2) compound 5-(4-chlorostyryl)-2-methoxycarbonylcyclopentanone
[0039] Add 142.0g p-chlorobenzaldehyde (99.0%) and 145.0g formula (1) compound 2-methoxycarbonylcyclopentanone into a 1L four-necked flask, then add 500ml methanol, heat up to reflux for 30min until completely dissolved, cool After reaching room temperature, 17.4 g of methanol solution of 28% sodium methoxide was added dropwise within 20 minutes. During the reaction, heat was released, cooled with appropriate ice water, and a light yellow solid precipitated after the drop was completed. Stir at 35°C for 4 hours, and then Cool to 5 DEG C and filter with suction, rinse the solid obtained by suction with 50ml of cold methanol, and dry to obtain a light yellow solid 5-(4-chlorostyryl)-2-methoxycarbonylcyclopentanone (that is, formula (2 ) compound) 258.0g, it...
Embodiment 2
[0053] The present embodiment provides a kind of preparation method of metconazole, concrete implementation is as follows:
[0054] (a) Preparation of formula (2) compound 5-(4-chlorostyryl)-2-methoxycarbonylcyclopentanone
[0055] Add 284.0g p-chlorobenzaldehyde (99.0%) and 290.0g formula (1) compound 2-methoxycarbonylcyclopentanone into a 2L four-neck flask, then add 1000ml methanol, heat and reflux for 30min until completely dissolved, and cool to At room temperature, dropwise add 37.0 g of methanol solution of 28% sodium methoxide within 20 minutes. Heat is released during the reaction. Cool with appropriate ice water. After dropping, a light yellow solid precipitates. Keep stirring at 35°C for 4 hours, then cool Suction filtration at 5°C, rinse the solid obtained by suction filtration with 50ml of cold methanol, and dry to obtain a light yellow solid 5-(4-chlorostyryl)-2-methoxycarbonylcyclopentanone (formula (2) Compound) 514.9g, its purity is 97.6%, the yield is 95.0% ...
Embodiment 3
[0069] The present embodiment provides a kind of preparation method of metconazole, concrete implementation is as follows:
[0070] (a) Preparation of formula (2) compound 5-(4-chlorostyryl)-2-methoxycarbonylcyclopentanone
[0071] Add 71.0g p-chlorobenzaldehyde (99.0%) and 72.5g formula (1) compound 2-methoxycarbonylcyclopentanone into a 500ml four-neck flask, then add 250ml methanol, heat and reflux for 30min until completely dissolved, and cool to At room temperature, 8.68g of methanol solution with a concentration of 28% sodium methoxide was added dropwise within 20 minutes. During the reaction, heat was released, cooled with appropriate ice water, a light yellow solid precipitated after dropping, kept stirring at 30°C for 4 hours, and then cooled Suction filtration at 5°C, the solid obtained by suction filtration was rinsed with 30ml of cold methanol, and dried to obtain a light yellow solid 5-(4-chlorostyryl)-2-methoxycarbonylcyclopentanone (that is, the formula (2) Com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com