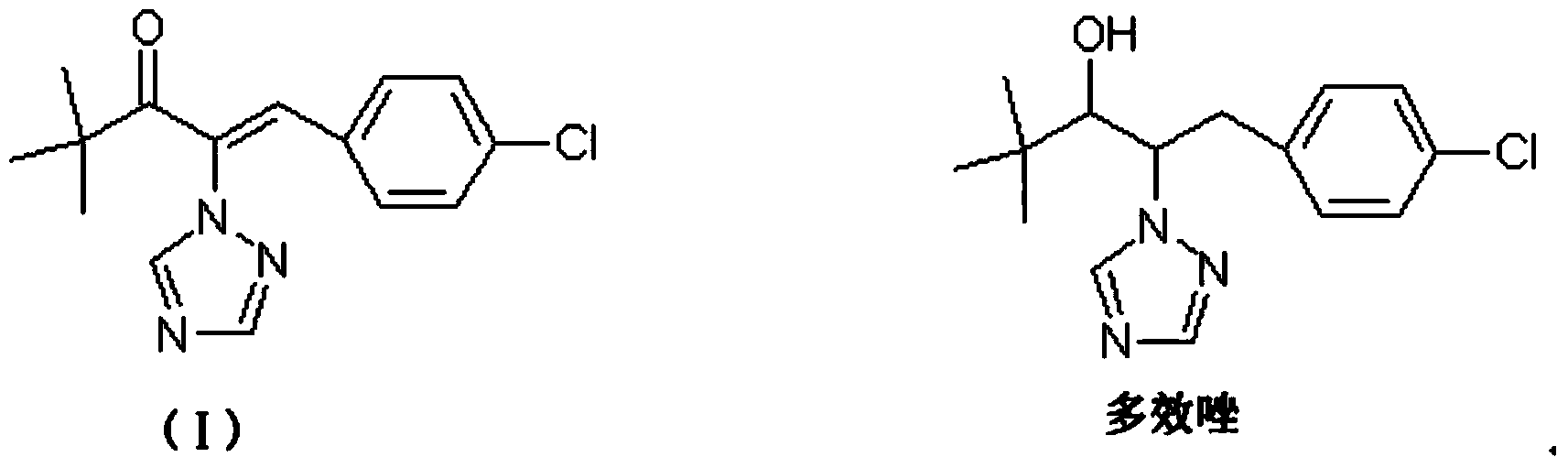Preparation method for paclobutrazol
A technology of paclobutrazol and reaction, which is applied in the direction of organic chemistry, can solve the problems of large environmental pollution, etc., and achieve the effect of no environmental pollution and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
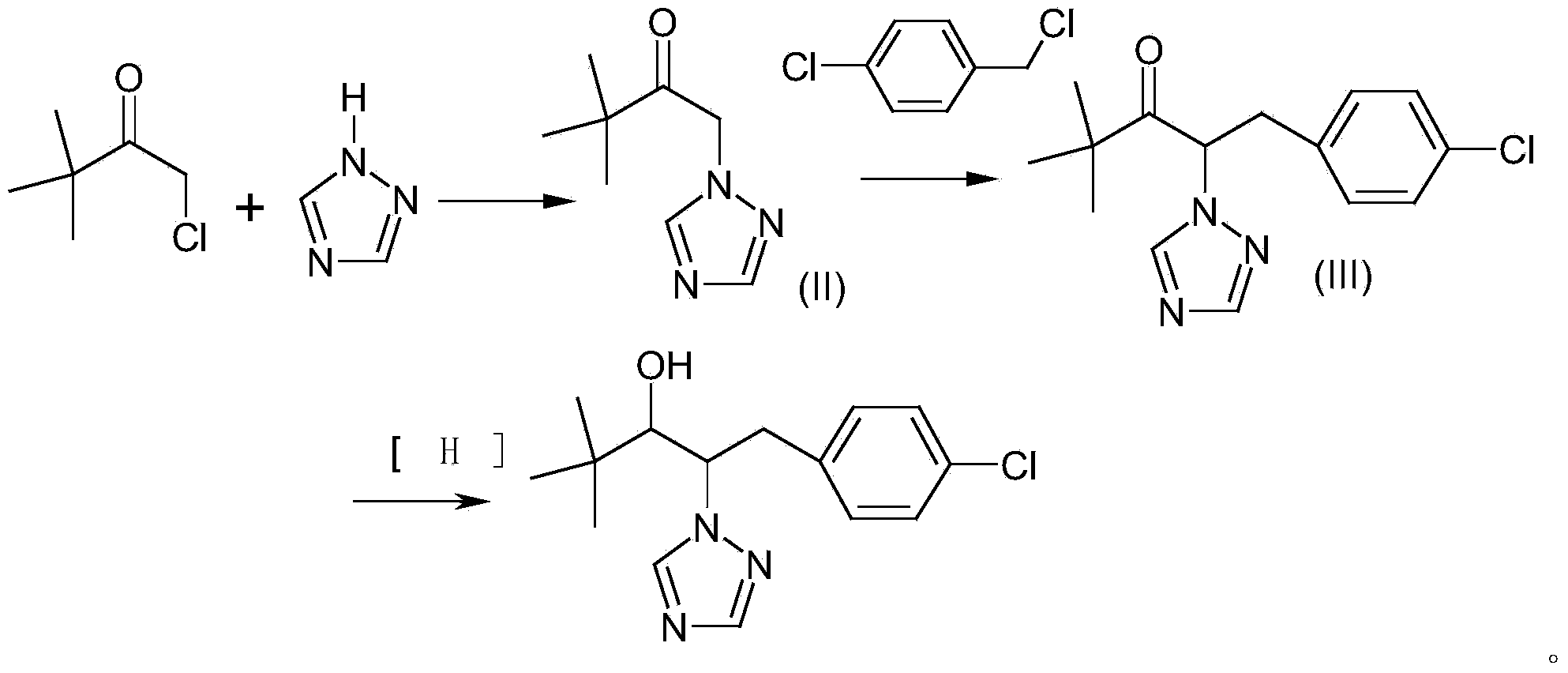
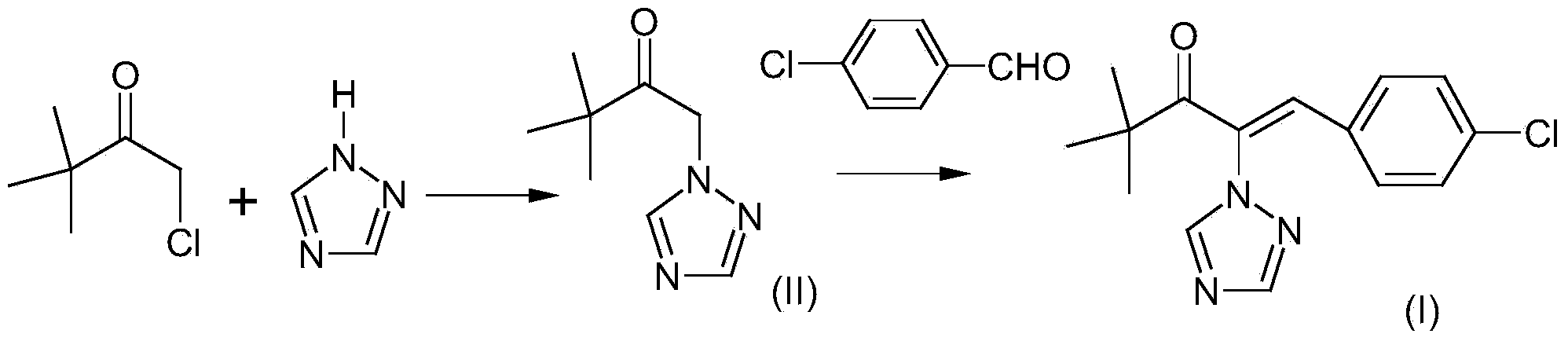
[0026] This embodiment provides a preparation method of a compound represented by formula (I), the specific implementation is as follows:
[0027] Add 7.99g (0.11mol) of 1,2,4-triazole, 8.45g (0.06mol) of potassium carbonate and 45g of ethanol to the reaction flask, heat to 60°C, then add 14.15g (0.1mol) dropwise over 1 hour ) α-chloropinacolone into the reaction bottle, after the dropwise addition, react at 60°C for 3 hours, and then take a sample for GC analysis. When the α-chloropinacolone is less than 1%, the reaction is terminated. The reaction liquid is cooled and filtered to recover potassium carbonate, after the filtrate recovers ethanol under normal pressure, the residue 18g is the compound represented by formula (II), then add 60g toluene, 13.7g (0.095mol) p-chlorobenzaldehyde, 1g trichlorobenzaldehyde to the filtrate Ethylamine, heated to 120°C, dehydrated with toluene reflux for 4 hours, sampled for GC analysis, when the p-chlorobenzaldehyde was less than 1%, the r...
Embodiment 2
[0029] The present embodiment provides a kind of preparation method of paclobutrazol, concrete implementation is as follows:
[0030] Add 90g of methanol, 30g (0.094mol) of the compound represented by formula (I) and 16.21g (0.3mol) of ammonium chloride to the reaction flask, stir and heat to 40°C, add 3.64g (0.15mol) in batches within half an hour ) magnesium powder, then react at 50°C for 1 hour, and absorb the ammonia gas released during the reaction with water. Sampling GC analysis formula (I) after the compound is less than 1%, the material is filtered to remove magnesium chloride, after the filtrate reclaims part of methanol, cooling and crystallization, filtration and drying, to obtain 26.2g off-white solid, which is paclobutrazol, content 96.2%. The rate is 91.4%.
Embodiment 3
[0032] The present embodiment provides a kind of preparation method of paclobutrazol, concrete implementation is as follows:
[0033] Add 120g of ethanol, 30g (0.094mol) of the compound represented by formula (I) and 21.61g (0.4mol) of ammonium chloride to the reaction flask, stir and heat to 50°C, add 4.85g (0.2mol) in batches within half an hour ) magnesium powder, then react at 60°C for 2 hours, and absorb the ammonia gas released during the reaction with water. Sampling GC analysis formula (I) after the compound is less than 1%, the material is filtered to remove magnesium chloride, after the filtrate reclaims part of the ethanol, cooling and crystallization, filtering and drying, obtain 26.4g off-white solid, which is paclobutrazol, content 96.1%. The rate is 91.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com