Half-path charge complementary type chiral self-assembled short-peptide nanometer biomedical material and application
A self-assembled short peptide, complementary technology, applied in the fields of medical science, peptides, absorbent pads, etc., can solve problems such as instability, achieve the effects of secondary structure stabilization, prolonged action time, and rapid hemostasis wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] When X is Glu and Y is Arg, the sequence of half-range charge complementary chiral short peptide ①D-P9-1 is as follows:
[0039] D-P9-1: Ac-Pro D -Glu D -Phe D -Arg D -Phe D -Asn D -Phe D -Gln D -Pro D -NH 2 . Half-range charge-complementary chiral short peptide ①Synthesis of D-P9-1: Ac is the acetylated N-terminal, NH 2 For amidated C-terminus
[0040] 1. Materials
[0041] Fmoc-Pro-OH(N-fluorenylmethoxycarbonyl-proline), Fmoc-Glu(OtBu)-OH(N-fluorenylmethoxycarbonyl-o-tert-butyl-glutamic acid), Fmoc-Phe-OH (N-fluorenylmethoxycarbonyl-phenylalanine), Fmoc-Arg(pbf)-OH(N-fluorenylmethoxycarbonyl-2,2,4,6,7-pentamethyldihydrobenzofuran- 5-sulfonyl-arginine), Fmoc-Asn(Trt)-OH (N-fluorenylmethoxycarbonyl-N-trityl-asparagine), Fmoc-Gln-OH (N-fluorenylmethoxycarbonyl Acyl-Glutamine), Rink Amide-MBHA Resin.
[0042] HBTU (O-benzotriazol-1-yl-N, N, N, N-tetramethylurine hexafluorophosphate) and HOBT (1-hydroxybenzotriazole) were purchased from Shanghai Keyept Bioch...
Embodiment 2
[0058] High-performance liquid chromatography and mass spectrometry detection and three-dimensional molecular model drawing of the half-range charge-complementary chiral self-assembled short peptide ①D-P9-1 of the present invention
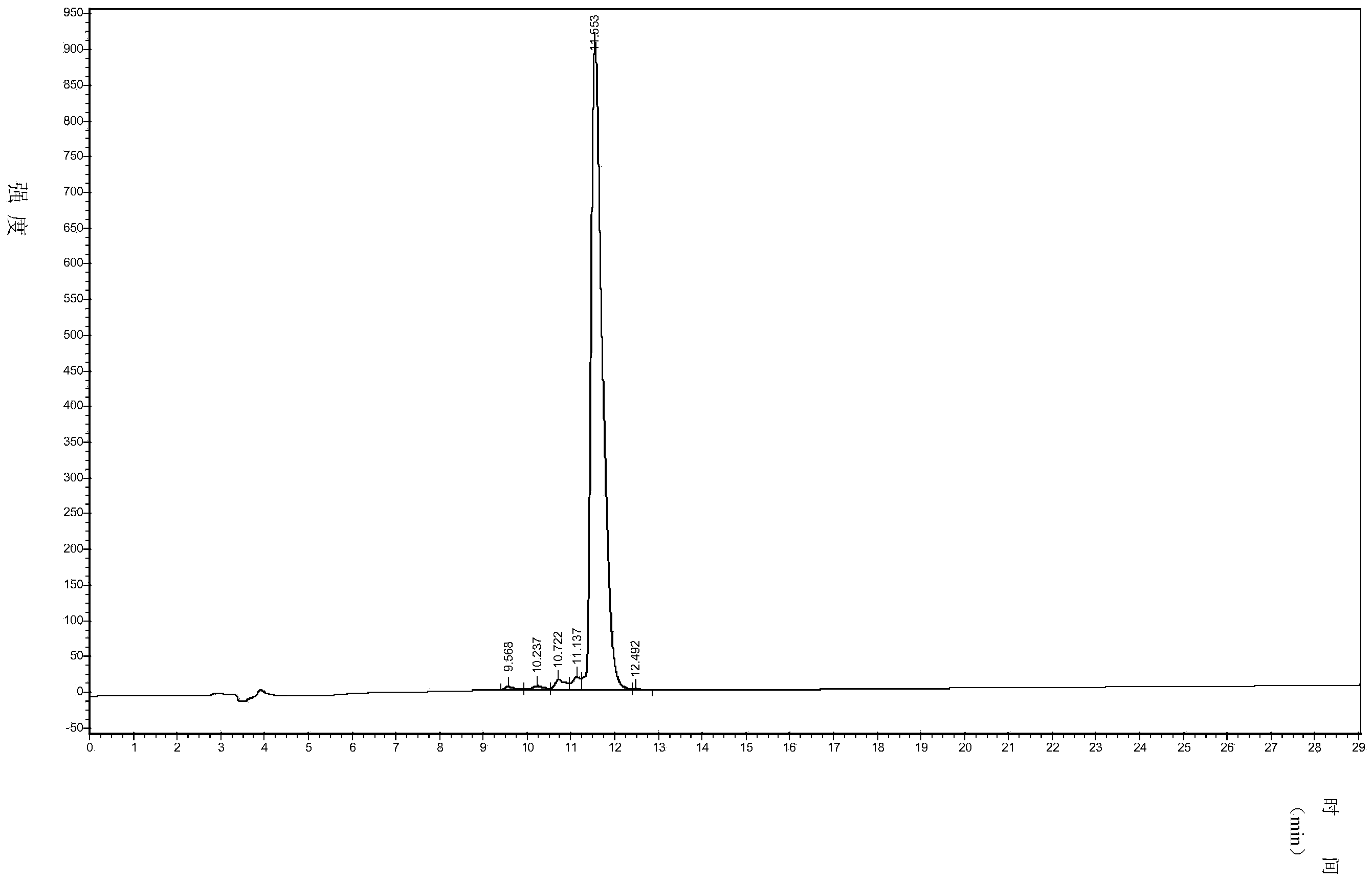
[0059] The half-course charge-complementary chiral self-assembled short peptide ①D-P9-1 prepared in Example 1 was detected by high performance liquid chromatography, and the detection results are shown in figure 1 ,according to figure 1 The results in determined that its purity reached 95.5%.
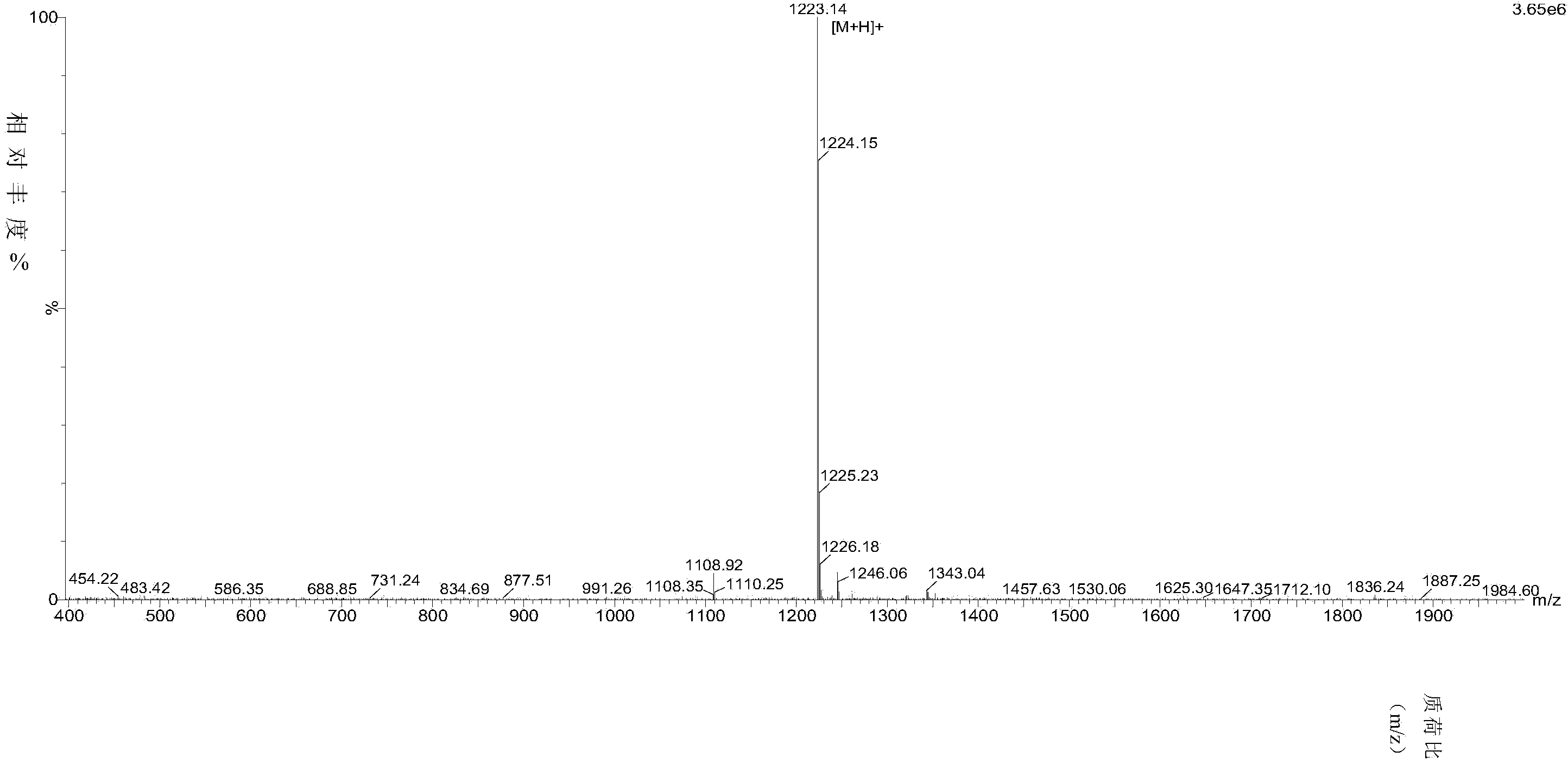
[0060] The half-range charge-complementary chiral self-assembled short peptide ①D-P9-1 prepared in Example 1 was detected by mass spectrometry, and the detection results are shown in figure 2 , the result showed that its molecular weight was 1222.38.
[0061] The half-range charge complementary chiral self-assembled short peptide ①D-P9-1 prepared in Example 1 was used to draw a schematic diagram of a three-dimensional molecular model based on the princip...
Embodiment 3
[0063] Detecting the secondary structure of the half-range charge-complementary chiral self-assembled short peptide ① D-P9-1 of the present invention using a circular dichroism spectrometer
[0064] 1. Detection of half-range charge-complementary chiral self-assembled short peptides using circular dichroism ① Secondary structure changes of D-P9-1 in solutions with different peptide concentrations
[0065] Test sample preparation: Take the half-range charge-complementary chiral self-assembled short peptide ①D-P9-1 stored at 4°C and prepare it with Milli-Q ultrapure water to the concentrations: 0.05mg / ml, 0.1mg / ml, 0.2 mg / ml, 0.5 mg / ml and 1.0 mg / ml test sample solutions.
[0066] Experimental operation and instrument parameter setting: Applied photophysics CD spectrometer is used for CD spectrum. A 1mm quartz cuvette is used during the experiment. Before the test, a small amount of test sample should be used to rinse the quartz cuvette to reduce experimental errors. Accurately ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com