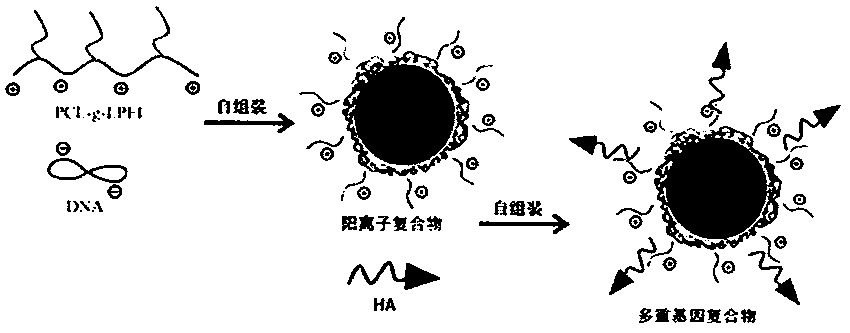
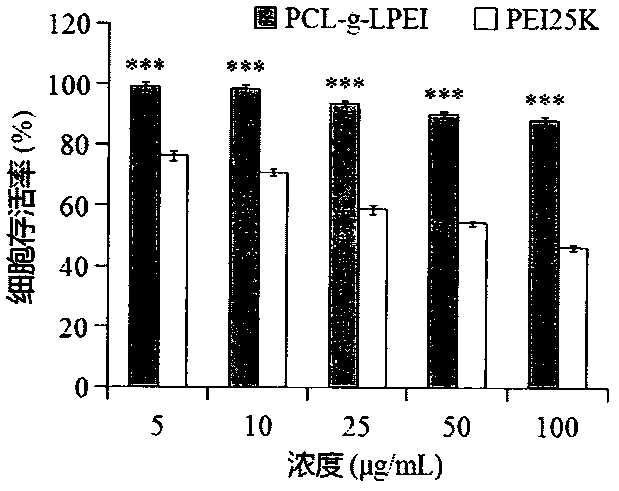
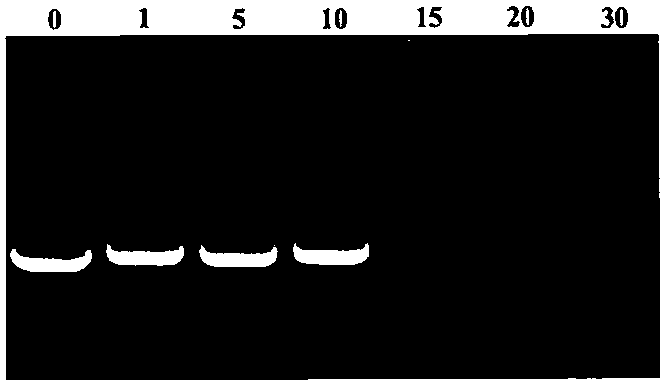
Novel cationic graft copolymer, and preparation method and application of multiple composite non-viral gene vector
A technology of graft copolymer and gene carrier, applied in the fields of biotechnology and pharmaceutical preparations, can solve the problems of high toxicity, coagulation of blood cells, low transfection efficiency, etc., and achieves low cytotoxicity, broad application prospects and high transfection efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Synthesis of cationic polymer PCL-g-LPEI
[0034] Using the ring-opening copolymerization method, ε-caprolactone (106.7mL, 1.0mol) and purified water (1mL, 55.6mmol) were injected into the three-necked flask, and the catalyst Sn(Oct) was added. 2 (1.8mL, 5mmol), react at 120°C for 2h, cool to 25°C, add appropriate amount of chloroform to dissolve, add to excess methanol for precipitation, filter the precipitate, and vacuum dry at 40°C for 4h to obtain polycaprolactone (PCL).
[0035] By infrared FT-IR and proton nuclear magnetic resonance spectroscopy 1 The structure of PCL is characterized by H NMR, and the spectrum is resolved as follows:
[0036] FT-IRIR(KBr): 3426.2(v OH (COOH)), 2946.2 (v as CH2 ), 2866.4(V s CH2 ), 1739.1 (v s C=O (COOH)), 1471.6(β CH2 ), 1419.8(β OH (COOH)), 1398.1(β oH (CH 2 OH)), 1295.4(v C-O-C ).
[0037] 1 H-NMR(300MHz, CDCl 3 ): δ1.35~1.41(H1, CH 2 ), δ1.62~1.68(H2, CH 2 ), δ2.28~2.31(H3, CH 2 ), δ4.05~4.07(H4, CH 2 ).
[0038] The...
Embodiment 2
[0044] Example 2: Synthesis of cationic polymer PCL-g-LPEI
[0045] Using the ring-opening copolymerization method, ε-caprolactone (106.7mL, 1.0mol) and purified water (1mL, 55.6mmol) were injected into the three-necked flask, and the catalyst Sn(Oct) was added. 2 (1.8mL, 5mmol), react at 120°C for 2h, cool to room temperature, add appropriate amount of chloroform to dissolve, add to methanol for precipitation, filter the precipitate, and dry under vacuum at 40°C to obtain polycaprolactone (PCL).
[0046] The yield of PCL was 58.3% and the molecular weight was 2392 Da.
[0047] Through the amide reaction, dissolve PCL (12.0g, 5mmol) in an appropriate amount of DMSO, add DCC (1.0g, 5mmol), NHS (0.6g, 5mmol), and react under nitrogen protection at 25°C for 12h, add LPEI (1.1g, 25mmol) , Stirring, dissolving, reacting at 80°C for 48h, filtering the solution, dialysis in deionized water for 72h (dialysis bag cut-off molecular weight 8000), freeze-drying to obtain PCL-g-LPEI.
[0048] The ...
Embodiment 3
[0049] Example 3: Synthesis of cationic polymer PCL-g-LPEI
[0050] Through the ring-opening copolymerization reaction, ε-caprolactone (106.7mL, 1.0mol) and purified water (0.9mL, 50.0mmol) were injected into the three-necked flask, and the catalyst Sn(Oct) was added. 2 (1.8mL, 5mmol), react at 120°C for 4h, cool to room temperature, add appropriate amount of chloroform to dissolve, add dropwise to excess methanol for precipitation, filter the precipitate, and dry under vacuum at 40°C for 4h to obtain polycaprolactone (PCL).
[0051] The yield of PCL is 51.8% and the molecular weight is 2996Da
[0052] Using amide reaction, dissolve PCL (15.0g, 5mmol) in an appropriate amount of DMSO, add DCC (1.0g, 5mmol), NHS (0.6g, 5mmol), and react under nitrogen protection at 25℃ for 12h, add LPEI (2.2g, 25mmol) , Stirring, dissolving, reacting at 80°C for 48h, filtering the solution, dialysis in deionized water for 72h (dialysis bag cut-off molecular weight 8000), freeze-drying to obtain PCL-g-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com