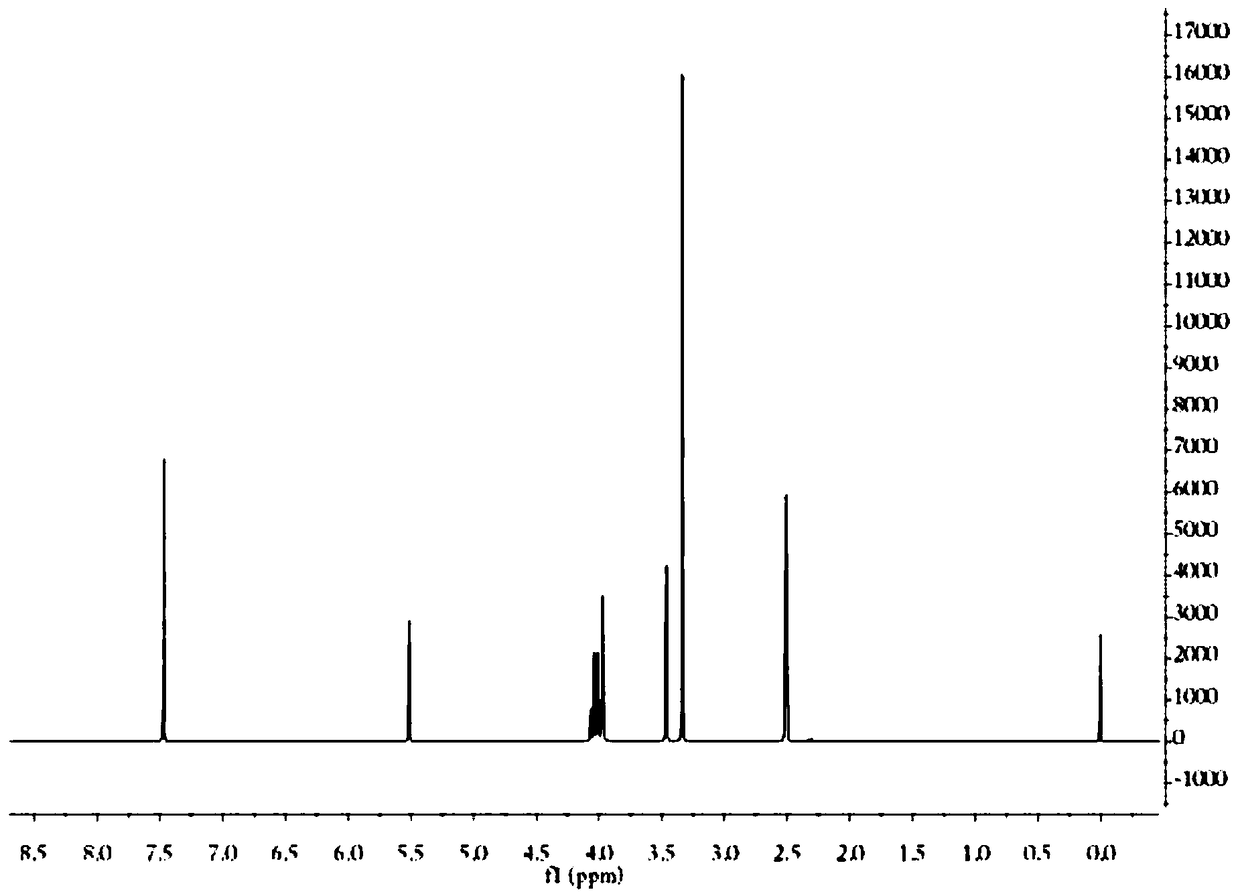
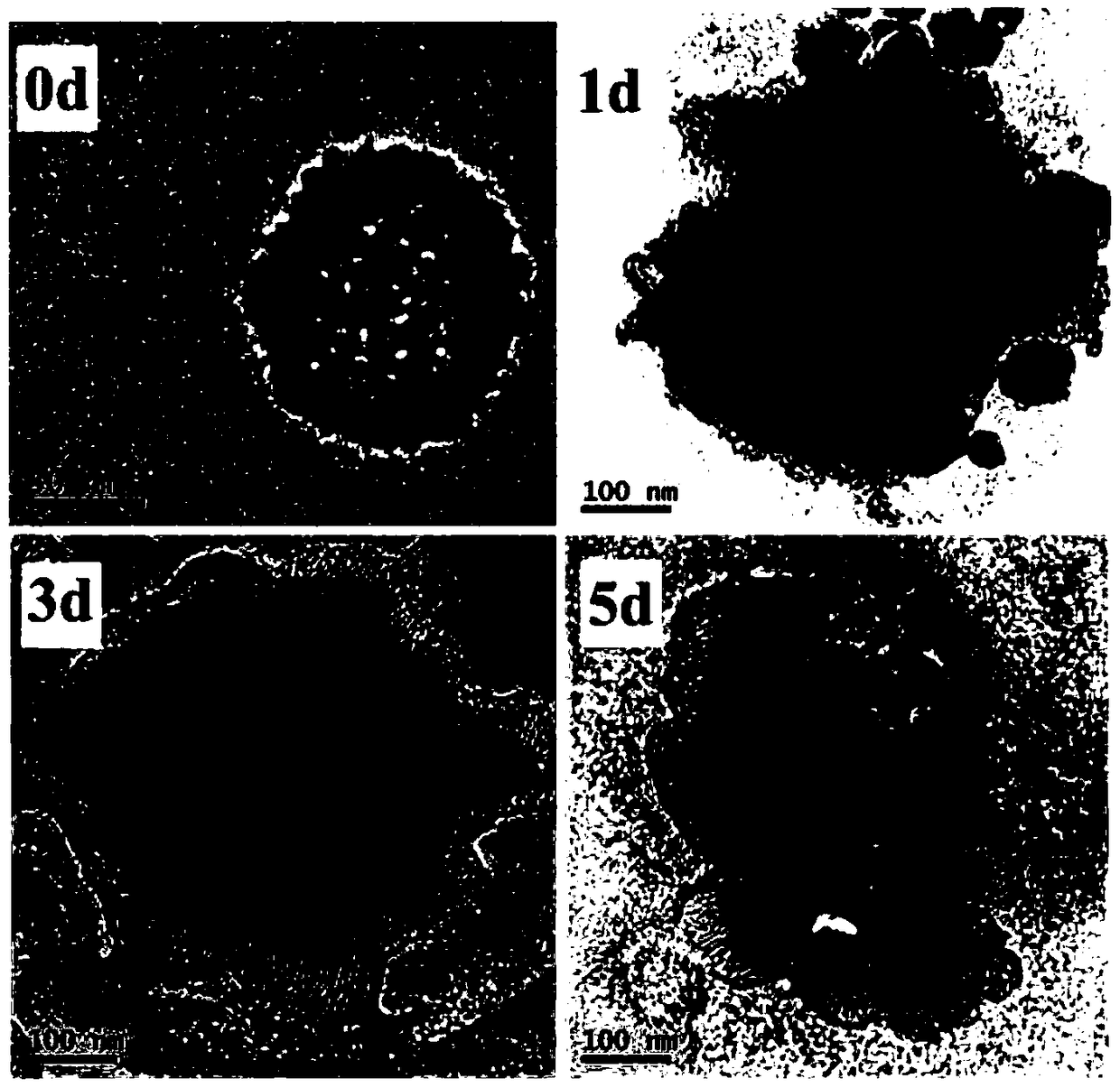
Preparation method of pH-responsive degradable hollow mesoporous organosilicon nanoparticle
A nanoparticle and organosilicon technology is applied in the field of preparation of pH-responsive degradable hollow mesoporous organosilicon nanoparticles, which can solve the problems of few types and limited applications, and achieve safe metabolism, increase loading, and avoid toxic and side effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Dissolve 20.0g of terephthalaldehyde, 80.0g of dibromoneopentyl glycol and 0.5g of p-toluenesulfonic acid in 300ml of toluene, reflux at 100°C for 9 hours, cool to room temperature, and filter to obtain a white powder that is Aldehyde groups for pH-responsive small molecules.
[0025] (2) Add 1.0g of triethylamine, 1.5g of 3-aminopropyltriethoxysilane coupling agent, and 0.5g of pH-responsive small molecules into 20ml of tetrahydrofuran, react at 50°C for 5h, and add 30ml of n-hexane after the reaction , the ammonium salt was removed through a neutral alumina column, and the bridging alkoxysilane precursor containing a pH-responsive group was obtained by rotary evaporation and drying.
[0026] (3) Dissolve 2.0 g of cetyltrimethylammonium bromide in 20 ml of water, then add 1.0 g of triethylamine and 2.0 g of tetraethylorthosilicate, and react at 80° C. for 1 hour to obtain a silica template; Then add 4.0g mixed silicon source, mixed silicon source is made up of 2.0...
Embodiment 2
[0028] (1) Dissolve 25.0g of terephthalaldehyde, 90.0g of dibromoneopentyl glycol and 1.5g of p-toluenesulfonic acid in 1000ml of benzene, reflux at 120°C for 16h, cool to room temperature, and filter to obtain a white powder that is condensate Aldehyde groups for pH-responsive small molecules.
[0029] (2) Add 2.0g of triethylamine, 2.5g of 3-isocyanatopropyltrimethoxysilane coupling agent, and 1.5g of pH-responsive small molecules into 20ml of tetrahydrofuran, react at 80°C for 8h, and add 60ml of n-hexane after the reaction , the ammonium salt was removed through a neutral alumina column, and the bridging alkoxysilane precursor containing a pH-responsive group was obtained by rotary evaporation and drying.
[0030] (3) Dissolve 5.0g of cetyltrimethylammonium bromide in 50ml of water, then add 3.0g of triethylamine and 5.0g of tetraethyl orthosilicate, and react at 100°C for 1.5h to obtain a silica template ; Then add 7.0g mixed silicon source, mixed silicon source is compo...
Embodiment 3
[0032] (1) Dissolve 45.0g of terephthalaldehyde, 170.0g of dibromoneopentyl glycol and 2.0g of p-toluenesulfonic acid in 1200ml of toluene, reflux at 110°C for 12.5h, cool to room temperature, and filter to obtain a white powder. Acetal groups for pH-responsive small molecules.
[0033] (2) Add 1.5g of triethylamine, 2.0g of 3-aminopropyltriethoxysilane coupling agent, and 1.0g of pH-responsive small molecules into 10ml of dimethyl sulfoxide, and react at 65°C for 6.5h. After the reaction Add 30ml of n-hexane, remove the ammonium salt through a neutral alumina column, and dry by rotary evaporation to obtain a bridged alkoxysilane precursor containing a pH-responsive group.
[0034] (3) Dissolve 3.5g of cetyltrimethylammonium bromide in 35ml of water, then add 2.0g of triethylamine and 3.5g of tetraethylorthosilicate, and react at 90°C for 1 hour to obtain a silica template; Then add 5.0g mixed silicon source, mixed silicon source is made up of 1.5g tetraethyl orthosilicate an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com