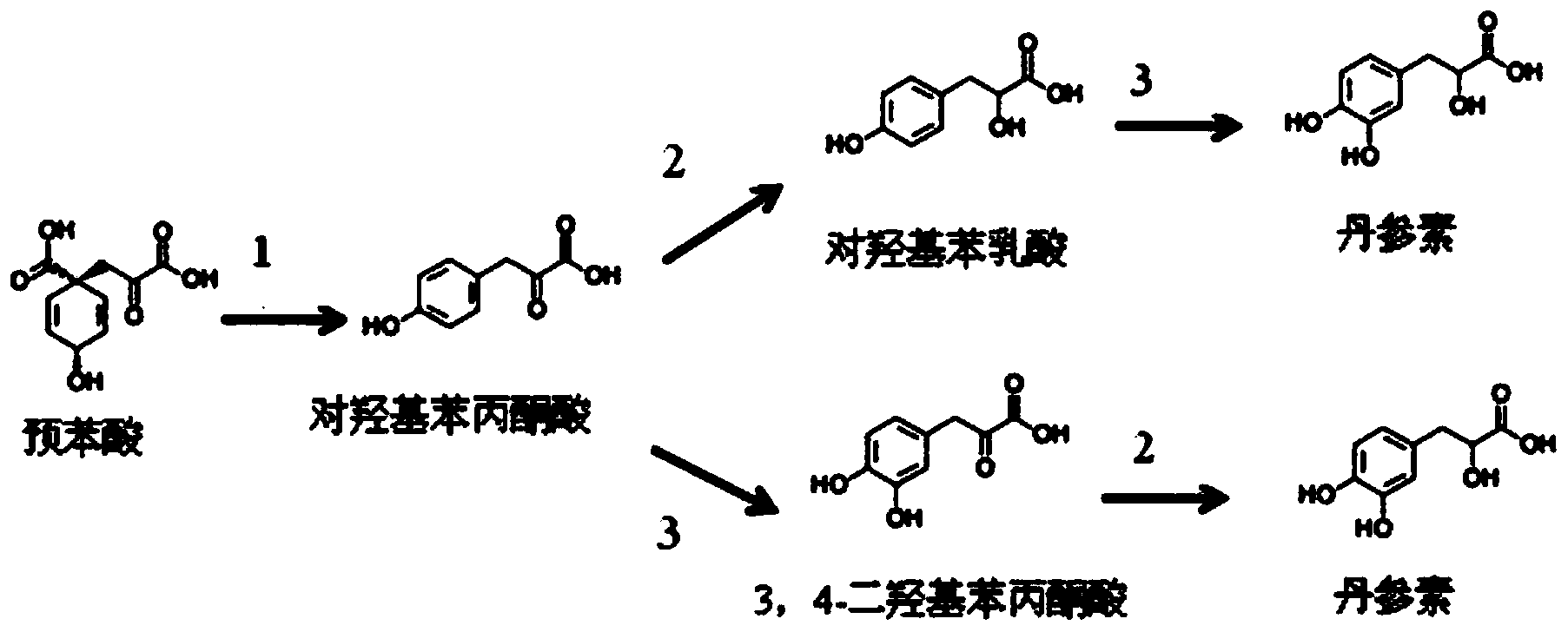
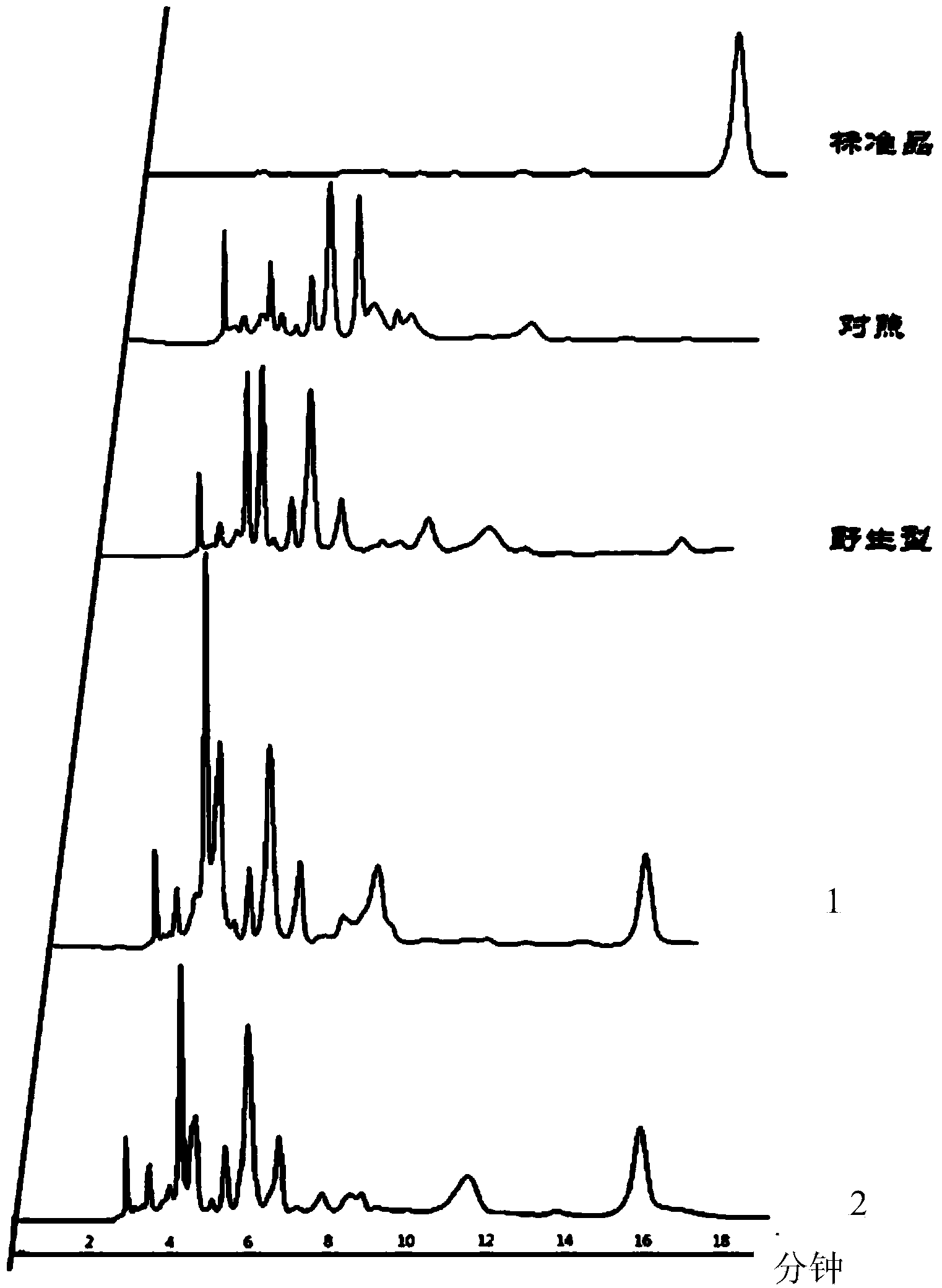
Biological production method of tanshinol
A production method and technology for Danshensu are applied in the field of biomedicine, and can solve the problems of unstable content, large loss of phenolic components, and high ethanol concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Screening of high-yield L-tyrosine strains
[0024] The Escherichia coli BW25113 obtained from the exchange with Japan (acquired by the National Institute of Genetics of Japan through gift or exchange in February 2009, the contact information of the provider is Yada 1111, Mishima, Shizuoka Prefecture 411-8540, Japan) Bacterial strains were subjected to (ultraviolet) mutagenesis culture in LB medium (10g / l NaCl, 10g / l peptone, 5g / l yeast extract), and candidate strains were isolated and fermented to detect L-tyrosine production.
[0025] Transfer 50uL of the preserved candidate strain to a test tube containing 5mL of pH=7.0 LB medium (and add the corresponding antibiotic: ampicillin 100ug / ml), culture at 37°C and 220rmp for 12h, and then transfer 500ul of the overnight cultured candidate strain to Transfer to 250mL shake flask containing 50ml pH=7.0 LB medium (and add corresponding antibiotic: ampicillin 100ug / ml), culture at 37°C, 220rmp. When OD0.6-0.8, add ...
Embodiment 2
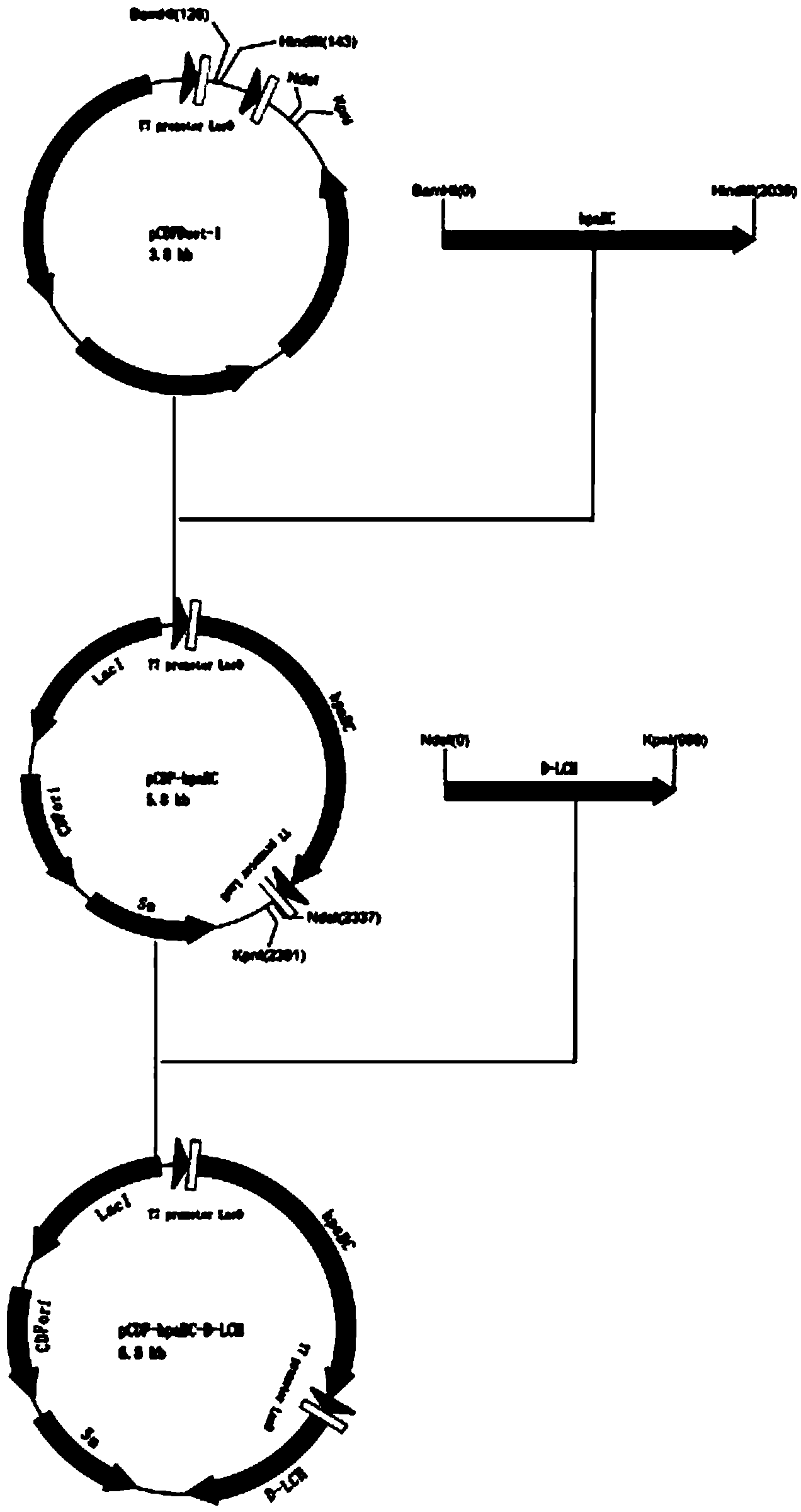
[0027] Example 2 Sequence acquisition of p-hydroxyphenylacetic acid meta-hydroxylase (hpaB, hpaC) and construction of pCDF-hpaBC Based on the p-hydroxyphenylacetic acid meta-hydroxylase (hpaB, hpaC) gene sequence of Escherichia coli BL21 (DE3) strain , design primers, and clone (that is, the gene sequence of p-hydroxyphenylacetic acid meta-hydroxylase provided by Escherichia coli BL21 (DE3) strain). Since the realization of the function of p-hydroxyphenylacetic acid meta-hydroxylase requires the completion of two genes, hpaB and hpaC, in the BL21 (DE3) genome, hpaB and hpaC genes are adjacent, so they are cloned and expressed together . The Escherichia coli BL21 (DE3) was provided by Beijing Quanshijin Biotechnology Co., Ltd., on the fourth floor of Building B-3, Zhongguancun Dongsheng Science and Technology Park, No. 66 Xixiaokou Road, Haidian District, Beijing. The gene sequence of p-hydroxyphenylacetic acid meta-hydroxylase (hpaB, hpaC) is shown in SEQ ID No.1 in the seque...
Embodiment 3
[0030] Example 3D-Lactate dehydrogenase (D-LCH) sequence acquisition and construction of pCDF-hpaBC-D-LCH expression vector
[0031] Using the D-LCH gene sequence of Lactobacillus plantarum (Lactobacillus plantarum) ATCC14917 as a reference, PCR primers were designed, and Lactobacillus plantarum CICC21419 (purchased at China Common Microorganism Culture Collection Management Center, No. 3, Yard 1, Beichen West Road, Chaoyang District, Beijing ) genome as a template for PCR reaction, and cloned into the pJET1.2 vector, sent for sequencing. Then ligated to the constructed pCDF-hpaBC vector to finally obtain an expression plasmid pCDF-hpaBC-D-LCH. The D-LCH gene sequence obtained by sequencing is shown in the sequence table SEQ ID NO.4, and the designed primers are as follows:
[0032] D-LCH-U (up), as shown in the sequence listing SEQ ID NO.5: GGGAATTC CATATG AAAATTATTGCCTATGCTGTAC (the underlined part is the NdeI restriction site); D-LCH-D (down), as shown in the sequence ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com