Timed controlled-release amlodipine capsule and preparation method thereof
An amlodipine and time-controlled technology, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the increased risk of toxicity, difficulty in taking medication, drug tolerance, etc. problems, to achieve the effect of improving compliance, reducing the difficulty of taking medicine, and increasing compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
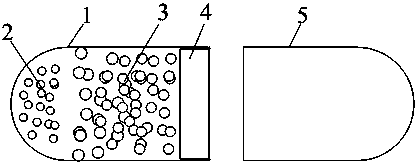
[0028]1) Preparation of non-permeable capsules: Dissolve non-permeable capsule materials and plasticizers in organic solvents at a concentration of 9.5-12.0% (W / V). Organic solvents are methanol, ethanol, dichloromethane, At least one of chloroform, ether, ethyl acetate, acetone, and petroleum ether; fill the prepared solution into gastric-soluble capsules, volatilize the solvent in the refrigerator or at room temperature, soak in water after the solvent is evaporated, and remove the gastric-soluble capsule. Capsule body, that is, obtain a non-permeable capsule body with one end closed and one end open;
[0029] 2) Preparation of amlodipine powder or tablet core: the drug can be drug powder or tablet; mix amlodipine and excipients (filler, stabilizer, disintegrant, lubricant) uniformly to obtain drug powder, or The drug powder is directly compressed to form a tablet core;
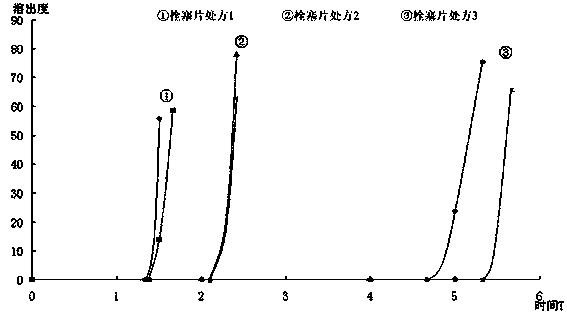
[0030] 3) Preparation of time-delayed embolic tablets: mix the blocking agent, porogen and lubricant ev...
Embodiment
[0033] a) Preparation of non-permeable capsules
[0034] Dissolve 12.5 g of ethyl cellulose (EC) in 100 ml of mixed solvent (dichloromethane: absolute ethanol: ethyl acetate = 4: 0.8: 0.2) to prepare an ethyl cellulose solution with a mass concentration of 125 g / L , and then pour it into the No. 1 capsule so that the liquid level is flush with the mouth of the capsule. Quickly put it in a 4°C refrigerator to evaporate the solvent, then soak the outer capsule body in water to obtain a non-permeable capsule body.
[0035] b) Preparation of drugs
[0036]
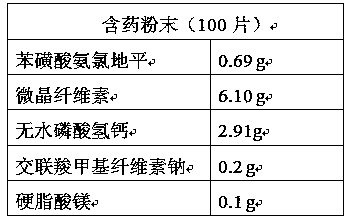
[0037] Mix amlodipine besylate, microcrystalline cellulose, calcium hydrogen phosphate anhydrous, sodium carboxymethyl starch and magnesium stearate evenly according to the prescription amount.
[0038] c) Preparation of time-delayed embolic tablets
[0039]
[0040]
[0041]
[0042] Weigh hypromellose E-50, direct-pressed lactose, and magnesium stearate, mix them evenly, and press them into tablets with a 5.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com