Cefquinome raw material and application of cefquinome preparation to preparing avian antibacterial medicine
A technology of cefquinome and antibacterial drugs, which is applied in the application field of cefquinome raw materials and its preparations in the preparation of antibacterial drugs for treating poultry, and can solve the lack of cefquinome data, the lack of clear clinical dosage, and the use of dosage cycles In order to avoid liver and kidney damage, to fully exert the effect of the medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Drug Sensitivity Test of Cefquinome Sulfate to Isolated Bacteria in Poultry (1)
[0016] 1.1 Drug sensitivity test materials
[0017] Cefquinome Sulfate Injection (Rip Biotech, batch number: 12122101), sample 1 (lincomycin hydrochloride + spectinomycin sulfate soluble powder (Pfizer, batch number: 12081901A)), sample 2 (ceftiofur for injection) Sodium (Pfizer, batch number: CVA130203), cefquinoxime sodium (Rip Bio, batch number: 13110901) ordinary culture broth, market isolation of drug-resistant bacteria, 96-well microtiter plate.
[0018] 1.2 Test method
[0019] 1.2.1 Preparation of bacterial solution Inoculate 2 relatively resistant strains of Escherichia coli isolated from chicken farms, 3 strains of resistant Escherichia coli isolated from duck farms, 1 strain of Salmonella isolated from chicken farms, and 2 strains of Salmonella isolated from duck farms The broth is cultured for 6-8 hours, and the test bacteria are diluted 1000 times with sterilized broth for...
Embodiment 2
[0030] Example 2: Drug sensitivity test of cefquinome sulfate to standard strains for poultry and isolated poultry strains
[0031] 1 The minimum inhibitory concentration test of Cefquinome Sulfate against avian standard Escherichia coli, Staphylococcus aureus, Pasteurella, and Streptococcus
[0032] Test method: Test strains are inoculated on an agar plate and incubated overnight at 37°C. Pick 1-2 single colonies and inoculate them in MH liquid culture medium. 5% bovine serum is added to the MH medium of Pasteurella and Streptococcus. Incubate at 37℃ for 18h to make the growth turbidity reach 5×10 8 -7.5×10 8 / mL. Dilute the original bacterial solution 1:1000 with MH liquid medium as the test bacterial solution.
[0033] Dilute the medicinal solution with MH liquid medium to 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25 μg / mL; add 2.0 mL each of the above-prepared bacterial suspension to be tested, and set it to 37 Cultivate for 48h in an incubator at ℃. The lowest concentration when there i...
Embodiment 3
[0042] Example 3: Pharmacokinetic test of cefquinome sulfate in broilers
[0043] Test purpose: to study the drug metabolism process of cefquinome sulfate in chickens by intramuscular injection, and the quantitative change of its main active ingredient cefquinome sulfate over time, to formulate a reasonable medication plan including dosage, treatment course, The route of administration, interval of administration, and withdrawal period provide important theoretical and practical basis.
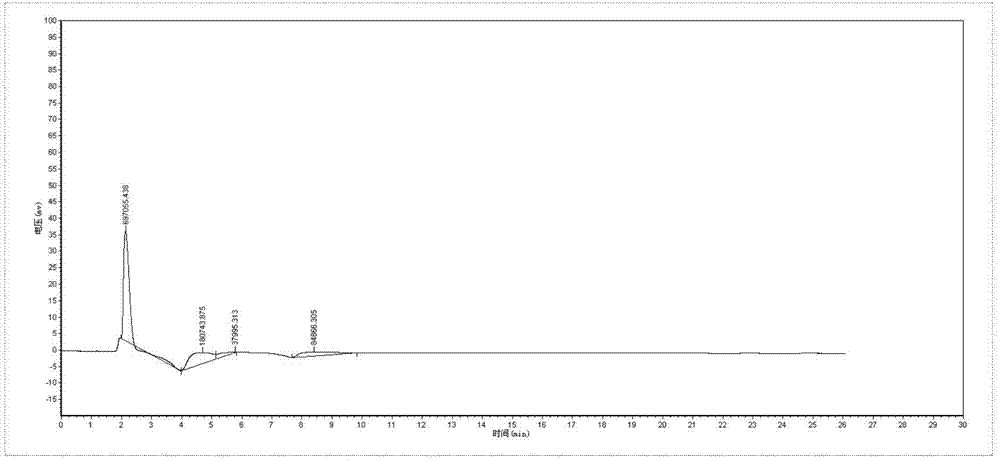
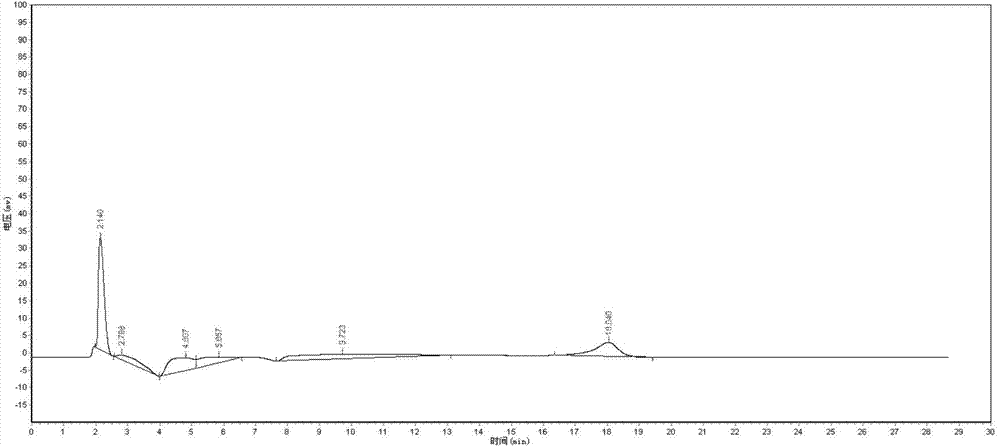
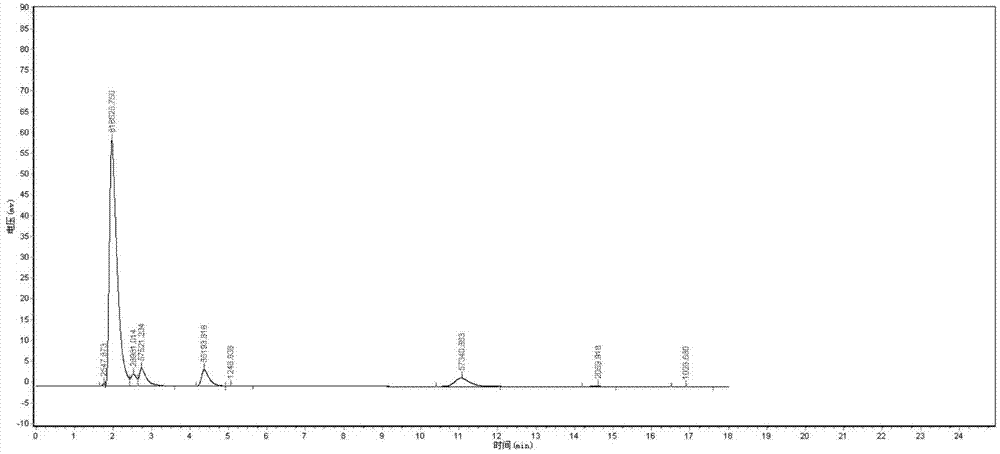
[0044] 1. Established a sensitive HPLC method for the determination of cefquinome sulfate in plasma
[0045] For the chromatogram of Cefquinome Sulfate in plasma, see the instructions attached Figure 1-Figure 3 .
[0046] 2. Pharmacokinetic test
[0047] 2.1 Methods of in vivo pharmacokinetics
[0048] The test chickens were weighed, and cefquinome sulfate injection was injected at a dose of 2 mg per kg body weight. Blood was collected at 15 min, 30 min, 60 min, 120 min, 180 min, 240 min, 300 min, 1200 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com