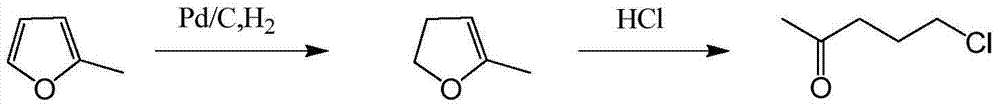
5-chloro-2-pentanone preparation method
A pentanone and cyclic chlorination technology, applied in the field of medicine and chemical industry, can solve the problems of cumbersome post-processing, large amount of waste water, complicated process, etc., and achieve the effects of reducing production cost, improving utilization rate and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Put 540kg of 2-methylfuran and 3kg of palladium-carbon catalyst (containing 5wt% palladium and 55wt% water) into the reactor, first replace it with nitrogen three times, stir and cool down to below 5°C, then replace it with hydrogen three times, and then continue For hydrogenation reaction, the pressure is controlled at 0.05-0.15MPa, and the temperature is between 5-15°C; when the content of 2-methyl-4,5-dihydrofuran reaches above 95% (GC value), the reaction ends and the addition is stopped. Hydrogen, the reaction time is about 8 hours. After replacing the hydrogen with nitrogen and filtering the catalyst in the reaction material in the separation tank, the filtrate is transferred to a halogenation metering tank for use. Add 2000kg of 15%-20% hydrochloric acid solution into the halogenation kettle, open the jacketed steam valve, heat up and stir to reach the reflux state, and when heated to 65°C, add dropwise 2-methyl-4,5 from the halogenation metering tank - Dihydrof...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com