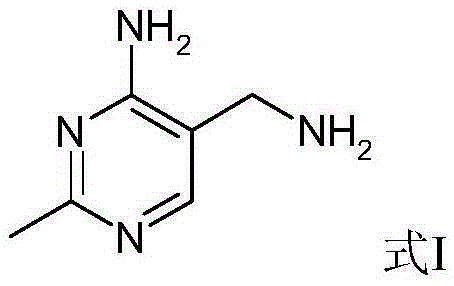
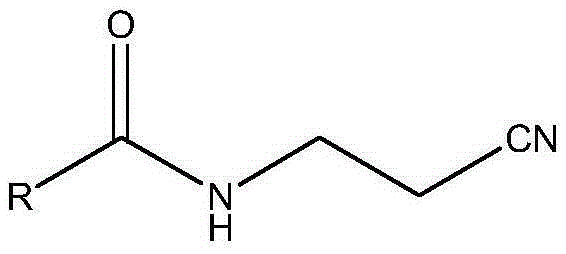
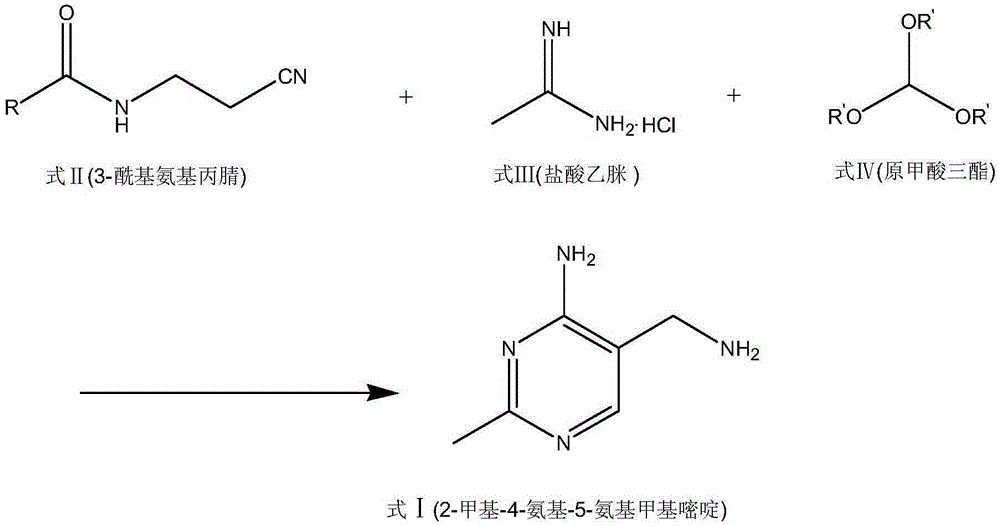
A convenient synthetic method for the preparation of 2-methyl-4-amino-5-aminomethylpyrimidine by one-step cyclization
A technique for the synthesis of aminomethylpyrimidine and its synthesis method, which is applied in the field of convenient synthesis of 2-methyl-4-amino-5-aminomethylpyrimidine, and can solve the difficulty of removing o-chloroaniline, unsatisfactory utilization rate, and consistency requirements High-level problems, to achieve the effects of production protection, saving raw material consumption, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of raw material 3-acylaminopropionitrile
[0039] Take 3-formylaminopropionitrile as an example:
[0040] Add 70 grams (1.0 moles) of 3-aminopropionitrile and 81.4 grams (1.1 moles) of ethyl formate to a 500 milliliter four-neck flask, react at room temperature at 20 ° C, and use gas phase detection to complete the reaction; after the reaction is completed, rectify to obtain 94.1 grams 3-Formylaminopropionitrile, gas phase purity 99.7%, yield 96.0%.
[0041] According to the above-mentioned method, replace the ethyl formate in embodiment 1 with ethyl acetate, n-ethyl propionate, n-butyrate ethyl ester, isobutyrate ethyl ester, ethyl benzoate or o-methylbenzoate ethyl ester respectively, 3-acetylaminopropionitrile, 3-propionylaminopropionitrile, 3-n-propylformylaminopropionitrile, 3-isopropylformylaminopropionitrile, 3-phenylformylaminopropionitrile can be obtained respectively Or 3-o-methylphenylformylaminopropionitrile. Reserve as a raw...
Embodiment 2
[0042] Embodiment 2: The preparation of 2-methyl 4-amino-5-aminomethylpyrimidine, taking 3-formamidopropionitrile as an example, the reaction formula is as follows:
[0043]
[0044] The synthesis steps are as follows:
[0045] Add 360 grams of isopropanol, 98 grams of 3-formamidopropionitrile, 104 grams of acetamidine hydrochloride, and 115 grams of trimethyl orthoformate into a 1000 milliliter glass reaction vessel, stir to dissolve; then add 12.2 grams of anhydrous zinc chloride ; Heating, the internal temperature rose to 85 ~ 90 ° C; stirring the reaction, the use of gas phase detection 3-acetamidopropionitrile reaction is complete.
[0046] After the reaction is completed, cool slightly, add a solution of 90 grams of sodium hydroxide and 200 grams of isopropanol to the obtained reaction liquid, react at 90-95 ° C, and use the liquid phase to detect the conversion of the intermediate until the reaction is completed. The purity and yield of the product 2-methyl-4-amino-5-...
Embodiment 3
[0049] As described in Example 1, the difference is that 112 grams of 3-acetamidopropionitrile is used to replace 98 grams of 3-formamidopropionitrile in Example 1 for the reaction. The preparation steps and conditions are the same as in Example 1, and the resulting product The purity and yield are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com