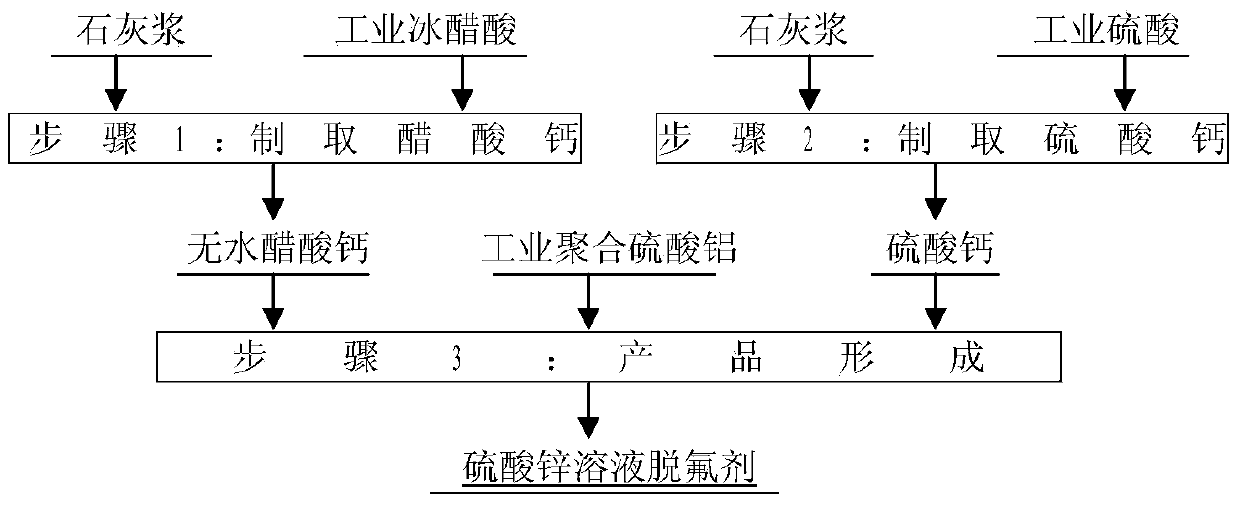
Method for preparing zinc sulfate solution de-fluorination agent
A technology of zinc sulfate solution and defluorinating agent, which is applied to the improvement of process efficiency, photographic technology, instruments, etc., can solve the problems of reduced defluorination efficiency, low calcium ion activity, and low defluorination efficiency, and achieve simplification of the defluorination process , simple production process and high defluorination efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
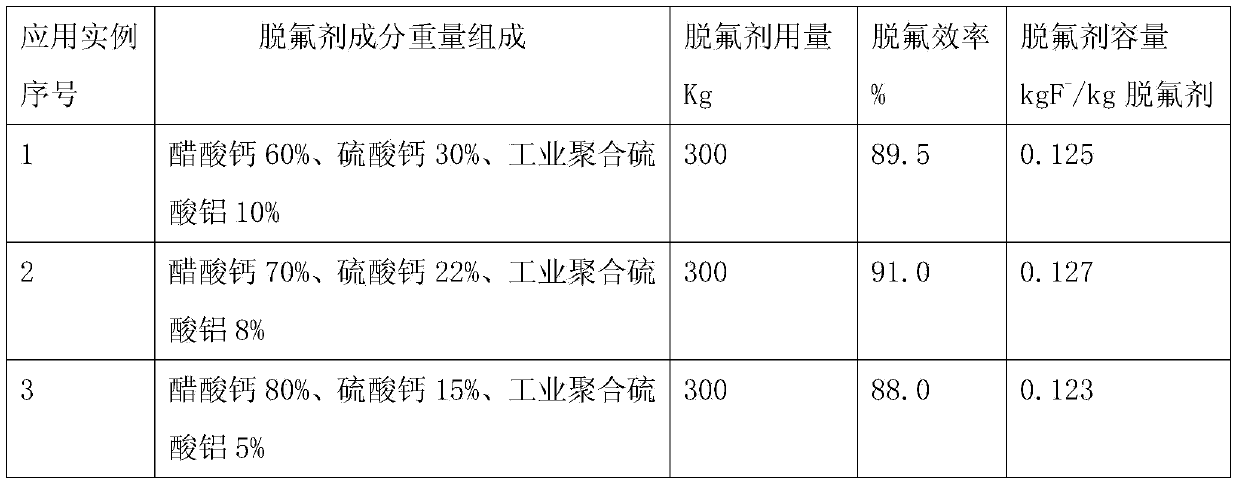
[0026] This embodiment is the first example of the method for preparing zinc sulfate solution defluorinating agent according to the present invention, comprising the following steps:
[0027] (1) Preparation of calcium acetate: Add 670kg of glacial acetic acid with a content of 99% at 20°C to 5m 3 React with 4250kg of lime slurry with a concentration of 20% in the enamel kettle for 30 minutes, and filter it with a filter press to obtain a filtrate that is a calcium acetate solution of 3.5m 3 , evaporated and concentrated to obtain 1951 kg of calcium acetate monohydrate, and dried calcium acetate monohydrate at 80 ° C for 8 hours to obtain 1750 kg of anhydrous calcium acetate;
[0028] (2) Preparation of calcium sulfate: Add 240kg of industrial sulfuric acid at 20°C to 5m 3 React with 1800 kg of lime slurry with a concentration of 10% in the enamel kettle for 60 minutes to generate calcium sulfate dihydrate solid, and dry the calcium sulfate dihydrate solid at 150 ° C for 4 ho...
no. 2 example
[0031] This embodiment is the second example of the method for preparing zinc sulfate solution defluorinating agent according to the present invention, comprising the following steps:
[0032] (1) Preparation of calcium acetate: Add 500kg of 99% glacial acetic acid at 30°C to 5m 3 React with 2070 kg of lime slurry with a concentration of 30% in the enamel kettle for 30 minutes, and filter it with a filter press to obtain a filtrate that is a calcium acetate solution of 1.9m3 , evaporated and concentrated to obtain 1460kg of calcium acetate monohydrate, and dried calcium acetate monohydrate at 90°C for 8 hours to obtain 1309kg of anhydrous calcium acetate;
[0033] (2) Preparation of calcium sulfate: Add 300kg of industrial sulfuric acid at 30°C to 5m 3 React with 1500 kg of lime slurry with a concentration of 15% in the enamel kettle for 60 minutes to generate calcium sulfate dihydrate solid, and dry the calcium sulfate dihydrate solid at 180 ° C for 4 hours to obtain 411 kg o...
no. 3 example
[0036] This embodiment is a third example of a method for preparing a zinc sulfate solution defluorination agent according to the present invention, comprising the following steps:
[0037] (1) Preparation of calcium acetate: Add 400kg of 99% glacial acetic acid at 35°C to 5m 3 React with 1250kg of lime slurry with a concentration of 40% in the enamel kettle for 30 minutes, and filter it with a filter press to obtain a filtrate that is a calcium acetate solution of 1.2m 3 , evaporated and concentrated to obtain 1166 kg of calcium acetate monohydrate, and dried calcium acetate monohydrate at 90 ° C for 8 hours to obtain 1046 kg of anhydrous calcium acetate;
[0038] (2) Preparation of calcium sulfate: add 382kg of industrial sulfuric acid at 35°C to 5m 3 React with 1430 kg of lime slurry with a concentration of 20% in the enamel kettle for 60 minutes to generate calcium sulfate dihydrate solid, and dry the calcium sulfate dihydrate solid at 200 ° C for 4 hours to obtain 523 kg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com