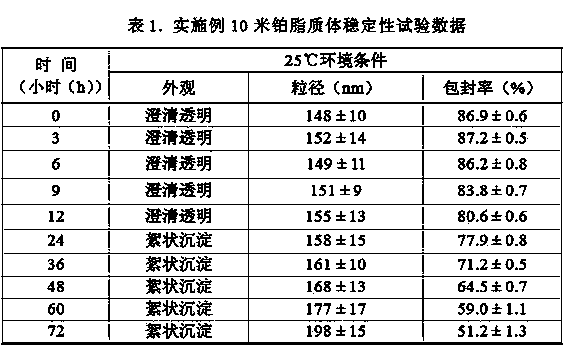
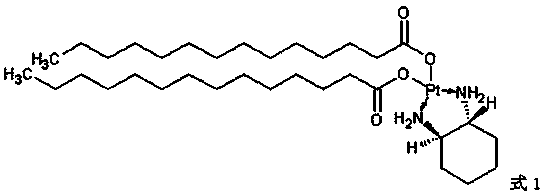
Miboplatin liposome and preparation method thereof
A liposome and miplatin technology, applied in the field of medicine, can solve the problems of lack of specificity and selectivity, toxic and side effects of cancer cells, and achieve the effects of drug stability, delayed release, and avoidance of leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of miplatin liposomes by film dispersion method: firstly mix 2.5 mL of chloroform and ethanol at a volume ratio of 9:1 as an organic solvent for later use, then weigh lecithin (700 mg), cholesterol (100 mg) and miplatin (70 mg ), add the organic solvent to dissolve, put the solution in a round bottom flask and rotate it to dryness under reduced pressure to form a phospholipid film, then add 100 mL of phosphate buffer solution to the round bottom flask, fully shake the flask to hydrate for 10- After 20 minutes, the lipid film was hydrated and fell off, the liposome liquid was taken out in a beaker, placed on a magnetic stirrer, stirred for 30-60 minutes, mixed evenly, and then sized through a 0.8 μm microporous membrane to obtain the final product. The particle size of miplatin liposomes prepared by this method is 270 ± 14 nm, and the encapsulation efficiency is about 62.9%.
Embodiment 2
[0034]Preparation of miplatin liposomes by film dispersion method: weigh dipalmitoylphosphatidylcholine (600 mg), cholesterol (100 mg) and miplatin (70 mg), add 3 mL of chloroform to dissolve, and place the solution in a circular Rotate the bottom flask and evaporate to dryness under reduced pressure to form a phospholipid film, add 80 mL of phosphate buffer solution to the flask, shake the flask fully for 10-20 min to hydrate the lipid film, and take out the liposome liquid in a beaker , placed on a magnetic stirrer, stirred for 30-60 min, mixed evenly, and sized through a 0.8 μm microporous membrane. The particle size of miplatin liposomes prepared by this method is 190 ± 10 nm, and the encapsulation efficiency is about 77.4%.
Embodiment 3
[0036] Preparation of miplatin liposomes by injection method: first mix 2.5 mL of chloroform and ethanol at a volume ratio of 9:1 as an organic solvent for later use, then weigh lecithin (700 mg), cholesterol (100 mg) and miplatin (70 mg ), added to the organic solvent to dissolve, and then injected the oil phase solution into 100 mL of sodium succinate buffer at a uniform speed using a syringe, stirred to remove the organic solvent, then milked evenly, and sized through a 0.8 μm microporous membrane, Promptly obtain miplatin liposome. The particle size of the liposome is 220 ± 18 nm, and the encapsulation efficiency can reach 70.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com