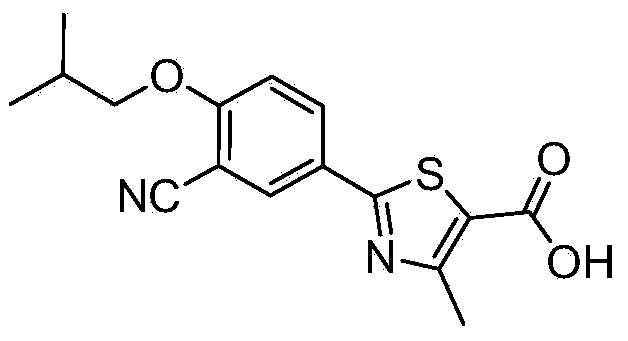
Preparation method of 2-(3-cyan-4-isobutoxyphenyl)-4-methylthiazole-5-formic acid A crystal
A technology of isobutoxyphenyl and methylthiazole, which is applied in the field of preparation of crystal form A of 2--4-methylthiazole-5-carboxylic acid, can solve the limitations, and does not disclose the mixed solvent system's ability to remove impurities, etc. problem, to achieve the effect of strong purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
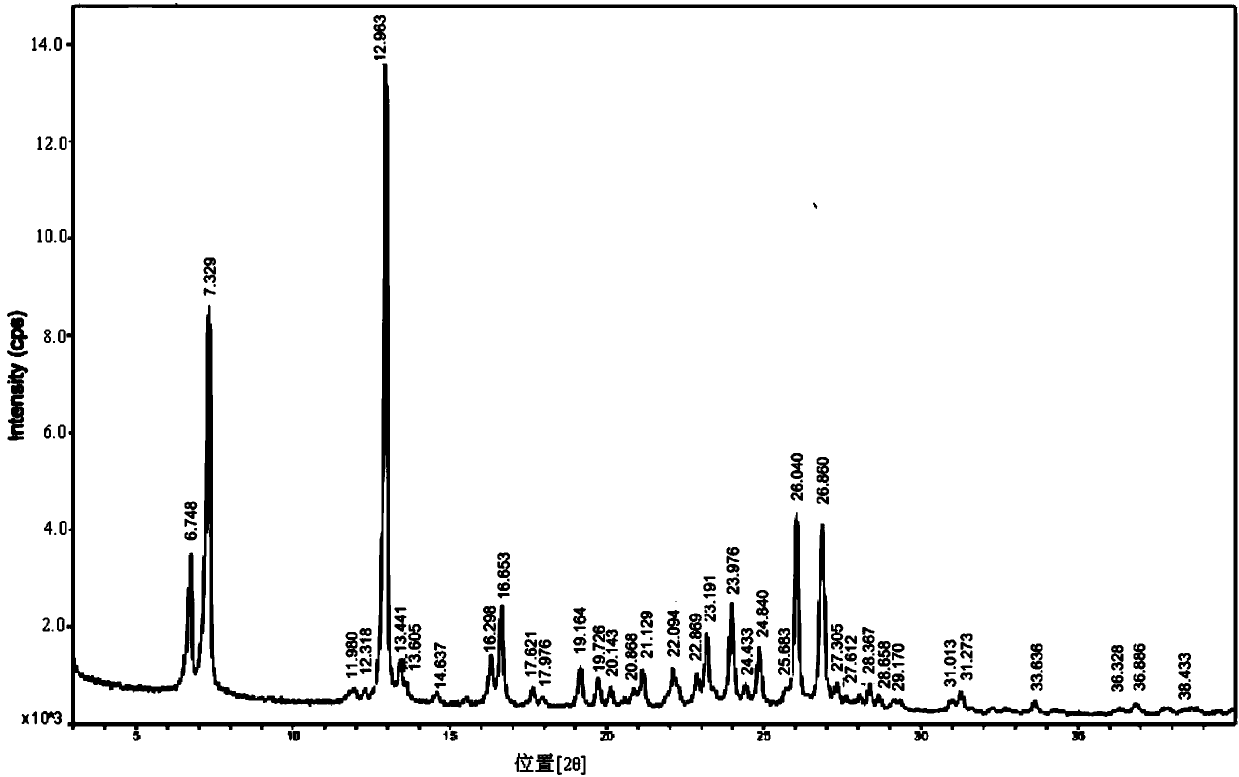
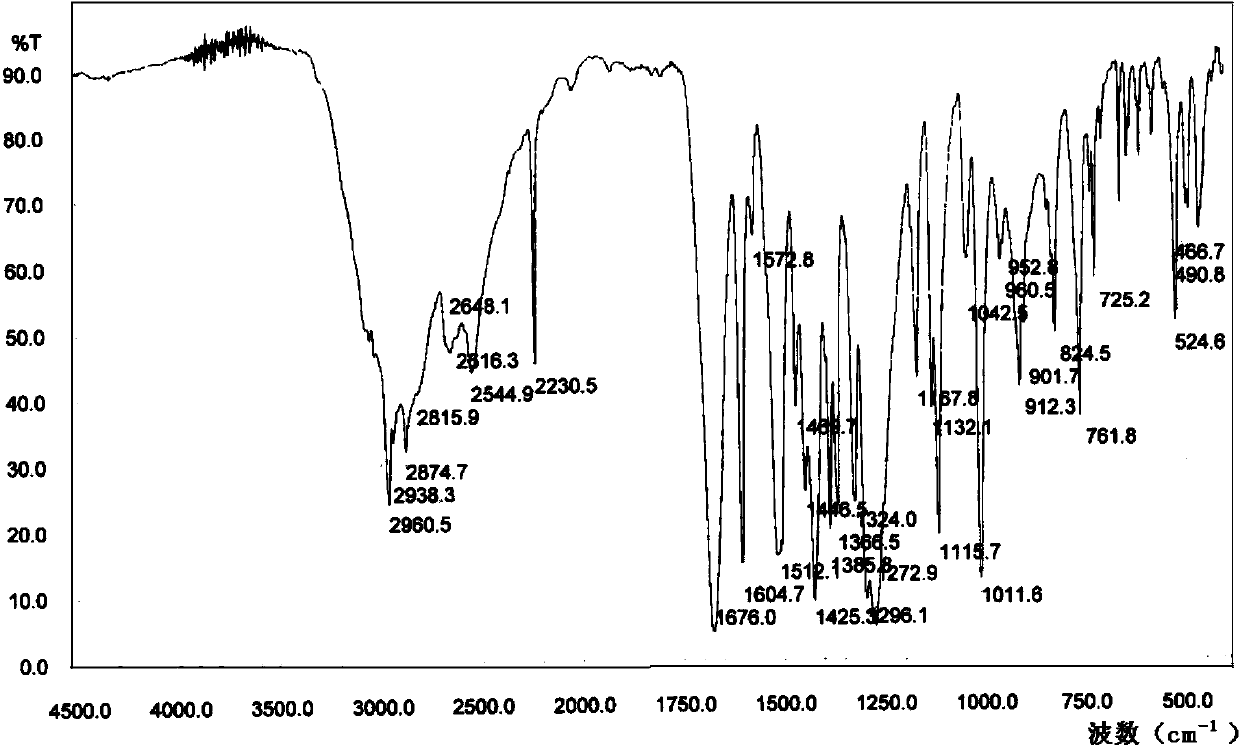
[0043] Add 6ml of n-propanol to 5.0g of febuxostat solid, heat to 85°C, add 50ml of isopropyl acetate, the solid is completely dissolved, then cool down to 0-5°C, filter the precipitated crystals and dry to obtain febuxostat Tan 4.24g, yield 84.8%, m.p=208.6~208.7℃. HPLC purity: 99.90%. Febuxostat ethyl ester was not detected. The febuxostat sample that is prepared is carried out XRPD and IR detection, and detection result is as follows figure 1 and figure 2 . The main peaks in the graph are shown in Table 1 below:
[0044] Table 1
[0045]
[0046] Depend on figure 1 As can be seen from Table 1, in the X-ray powder diffraction spectrogram of the sample, represented by reflection angle 2θ, it is roughly at 6.748, 7.329, 12.963, 13.441, 16.298, 16.653, 17.621, 19.164, 19.726, 20.868, 21.129, 22.094, 22.869, There are characteristic peaks at 23.191, 23.976, 24.433, 24.840, 25.683, 26.040, 26.860, 29.170, 31.013, 31.273, 36.686 and 38.433, which are basically consisten...
Embodiment 2
[0048] Add 40ml of isopropyl acetate to 5.0g of febuxostat solid, heat to reflux at 85°C, add 6ml of n-propanol, the solid is completely dissolved, then cool down to 20-25°C, filter the precipitated crystals and dry to obtain febuxostat 3.62 g, yield 72.4%, XRPD and IR showed that the obtained crystal was Form A (XRPD and IR detection results were the same as in Example 1), m.p=208.7-208.9°C. HPLC purity: 99.91%. Febuxostat ethyl ester was not detected.
Embodiment 3
[0050] Add 40ml of n-propyl acetate to 5.0g of febuxostat solid, heat to 95°C, add 2.5ml of n-propanol, the solid is completely dissolved, then cool down to -10~-5°C, filter the precipitated crystals and dry them to obtain febuxostat Buxostat 4.0g, yield 80.0%, XRPD and IR showed that the obtained crystals were crystal form A (XRPD and IR detection results were the same as in Example 1), m.p=208.5-208.6°C. HPLC purity: 99.93%. Febuxostat ethyl ester was not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com