Method for improving beta-cyclodextrin production capability of cyclodextrin glycosyltransferase by calcium ion binding site amino acid residue mutation
A technology of glucosyl and binding sites, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of separation and purification of unfavorable products, increase the production cost of cyclodextrin, etc., and achieve the effects of being beneficial to industrial production and improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: This example specifically illustrates the preparation of mutants A315R and A315D
[0026] (1) Site-directed mutation
[0027] Using rapid PCR technology, site-directed mutagenesis was carried out with the expression vector pST / cgt containing the wild CGTase gene as a template.
[0028] The mutation primers introducing the Arg315 codon are:
[0029] Forward primer: 5'-GCAGCCGATTAC CGC CAGGTGGATG-3', the underline is the mutant base,
[0030] Reverse primer: 5'-GTCATCCACCTG GCG GTAATCGGCTG-3', the underline is the mutant base;
[0031] The mutation primers that introduce the Asp315 codon are:
[0032] Forward primer: 5'-GCAGCCGATTAC GAC CAGGTGGATG-3', the underline is the mutated base.
[0033] Reverse primer: 5'-GTCATCCACCTG GTC GTAATCGGCTG-3', the underline is the mutated base.
[0034] The PCR reaction system is: 5×PrimeSTAR Buffer (Mg 2+ Plus) 10 μL, dNTPs (2.5 mM each) 4 μL, forward primer (10 μM) 1 μL, reverse primer (10 μM) 1 μL, template DN...
Embodiment 2
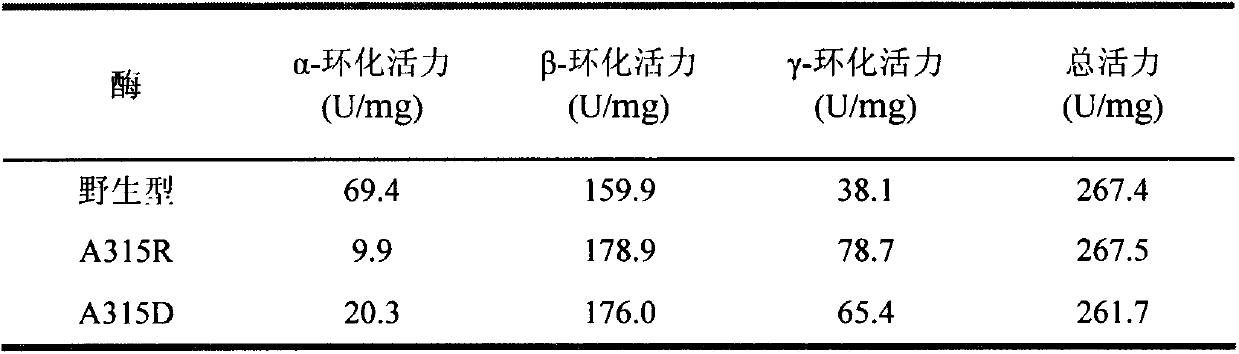
[0040] Embodiment 2: This example specifies enzyme activity analysis
[0041] (1) Determination of enzyme activity
[0042] Method for measuring α-cyclization activity by methyl orange method: Take 0.1 mL of appropriately diluted enzyme solution, add 0.9 mL of 1% (w / v) soluble starch solution prepared in advance with 50 mM phosphate buffer (pH 6.5) After reacting at 40°C for 10min, add 1.0mL of 1.0N hydrochloric acid to stop the reaction, then add 1.0mL of 0.1mM methyl orange solution prepared with 50mM phosphate buffer, keep warm at 16°C for 20min, and measure the absorbance at 505nm . One enzyme activity unit is defined as the amount of enzyme required to generate 1 μmol α-cyclodextrin per minute under the above conditions.
[0043] The method for measuring β-cyclization activity by phenolphthalein method: take 0.1 mL of appropriately diluted enzyme solution and add it to a test tube containing 0.9 mL of 1% (w / v) soluble starch solution prepared in advance with 50 mM phospha...
Embodiment 3
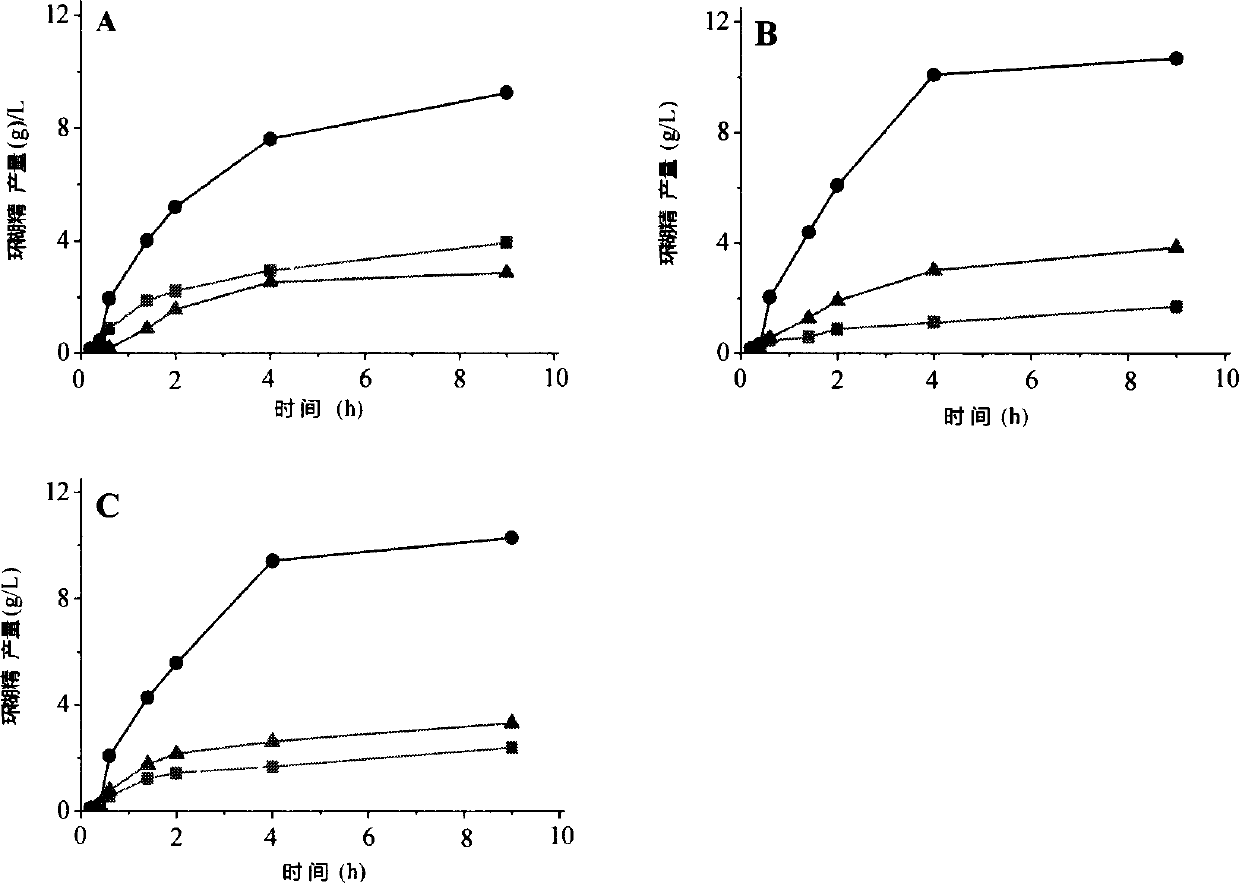
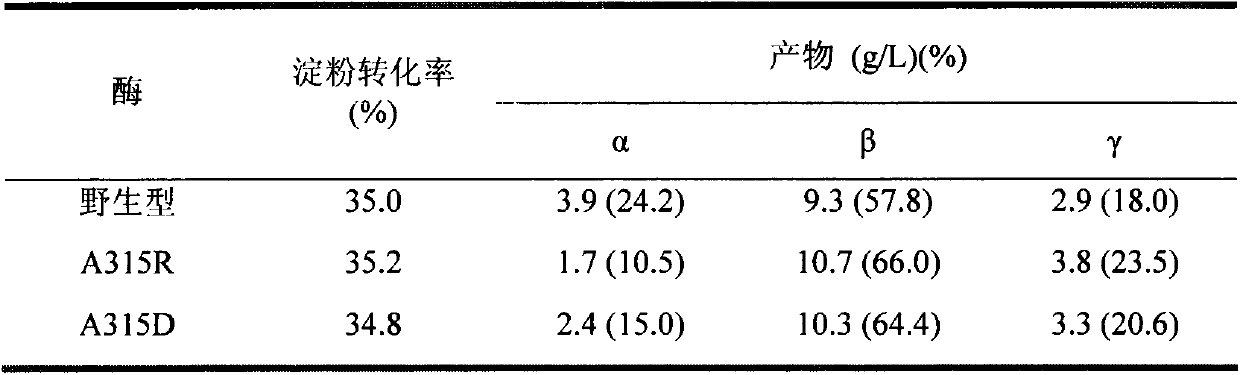
[0049] Example 3: This example specifically illustrates the use of HPLC to measure the amount of cyclodextrin produced
[0050] To prepare 5% (wet basis, water content 8%, w / v) maltodextrin (DE3) solution as substrate, 5g maltodextrin (DE3) was dissolved in 90mL sodium phosphate buffer (pH6.0), fixed Make up to 100mL, boil in boiling water for 30min. Add a certain amount of wild CGTase, mutants A315R and A315D to make the enzyme activity in the reaction system 1U / mL, place it at 50°C for 9 hours, sample 600 μL at intervals, boil the enzyme for 10 minutes, centrifuge at 12000rpm for 10 minutes, and take the supernatant 500 μL, add 5 μL glucoamylase (70 U / mL), saccharify at 30°C for 1 hour, boil for 10 minutes to inactivate, centrifuge at 12000 rpm for 30 minutes, take 20 μL of the supernatant and filter it through a 0.45 μm ultrafiltration membrane for HPLC analysis.
[0051] HPLC determination conditions are: Waters600 high performance liquid chromatograph (with differential ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com