Preparation method of 5alpha-androstanedion
A technology for androstanedione and compounds, which is applied in the field of preparation of 5α-androstanedione, can solve problems such as long steps and environmental pollution, and achieve the effects of low cost, easy availability of raw materials, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
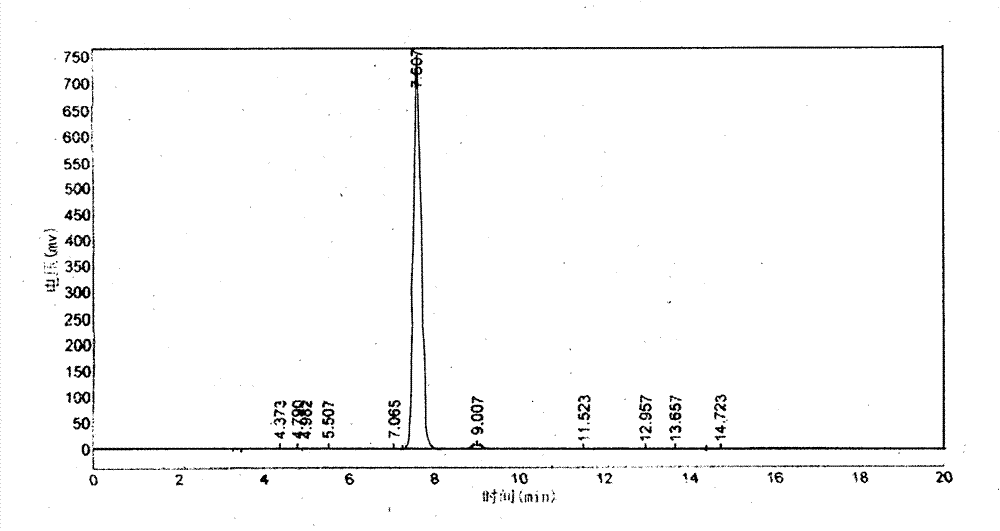
Image
Examples
Embodiment 1
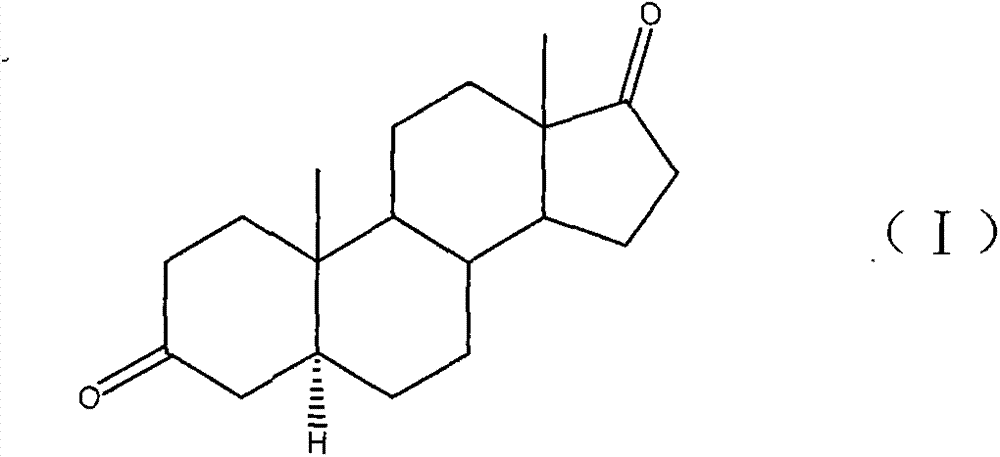
[0021] Example 1: The preparation method of this 5α-androstanedione adopts the following specific process steps, and the raw materials can be purchased from Danjiangkou Gongbio Co., Ltd.
[0022] (1) Reduction reaction:
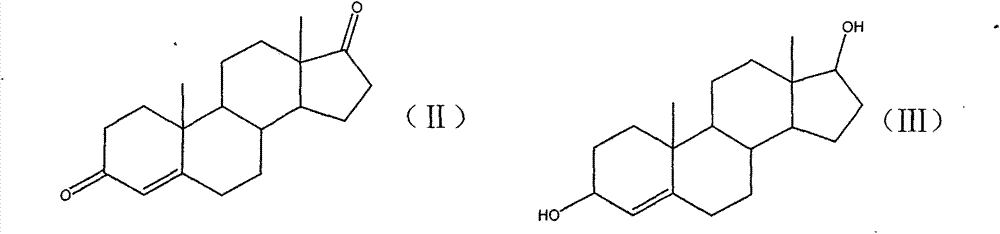
[0023] Add 20.0g of the compound of formula (II) and 200ml of methanol into the reaction flask, stir the dissolved material, and cool down to 5°C; add once every 10 minutes, and add 4.5g of potassium borohydride in 3 batches; , TLC (thin layer chromatography) detects that there is no raw material point in the reaction; add an appropriate amount of glacial acetic acid dropwise to adjust the pH to 7.0, steam the methanol under reduced pressure below 70 degrees, pour into 800ml water for water analysis, stir for 30 minutes, suction filter, and a large amount of solids Washed with water, dried at 70°C to constant weight to obtain 20.0 g of the compound of formula (III) in the form of white powder, with a yield of 100.0%.
[0024] (2) Hydrogenation reaction:
[...
Embodiment 2
[0028] Example 2: The preparation method of this 5α-androstanedione adopts the following specific process steps.
[0029] (1) Reduction reaction:
[0030] Add 20.0g of the compound of formula (II) and 240ml of methanol into the reaction flask, stir and dissolve the material, cool down to 0°C, add once every 10 minutes, add 5.0g of potassium borohydride in 3 batches, and keep warm for 2 hours after adding , TLC detects that there is no raw material point in the reaction, add an appropriate amount of glacial acetic acid dropwise to adjust the pH to 7.0, distill methanol under reduced pressure below 70 degrees, pour into 800ml water for water analysis, stir for 30 minutes, suction filter, wash the solid with a large amount of water, and dry at 70 degrees to Constant weight was obtained to obtain 20.0 g of the compound of formula (III) as a white powder, with a yield of 100.0%.
[0031] (2) Hydrogenation reaction:
[0032] Add 20.0g of the compound of formula (III) and 300ml of ...
Embodiment 3
[0035] Embodiment 3: The preparation method of this 5α-androstanedione adopts the following specific process steps.
[0036] (1) Reduction reaction:
[0037] Add 20.0g of compound of formula (II) and 100ml of ethanol into the reaction flask, stir and dissolve the material, cool down to 20°C, add once every 10 minutes, add 2.0g of sodium borohydride in 3 batches, and keep warm for 2 hours after adding , TLC detects that there is no raw material point in the reaction, add an appropriate amount of glacial acetic acid dropwise to adjust the pH to 7.0, evaporate ethanol under reduced pressure below 70 degrees, pour 800ml of water into water, stir for 30 minutes, suction filter, wash the solid with a large amount of water, and dry it at 70 degrees to Constant weight was obtained to obtain 20.0 g of the compound of formula (III) as a white powder, with a yield of 100.0%.
[0038] (2) Hydrogenation reaction:
[0039] Add 20.0g of the compound of formula (III) and 160ml of methanol i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com