Active oxygen free radical sensitive cyclodextrin material as drug delivery carrier and preparation method thereof
A delivery carrier and cyclodextrin technology, which is applied in the direction of non-active components of polymer compounds, can solve the problems of limitations and lack of materials, and achieve the effects of simple preparation methods, easy large-scale synthesis, and good in vivo biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
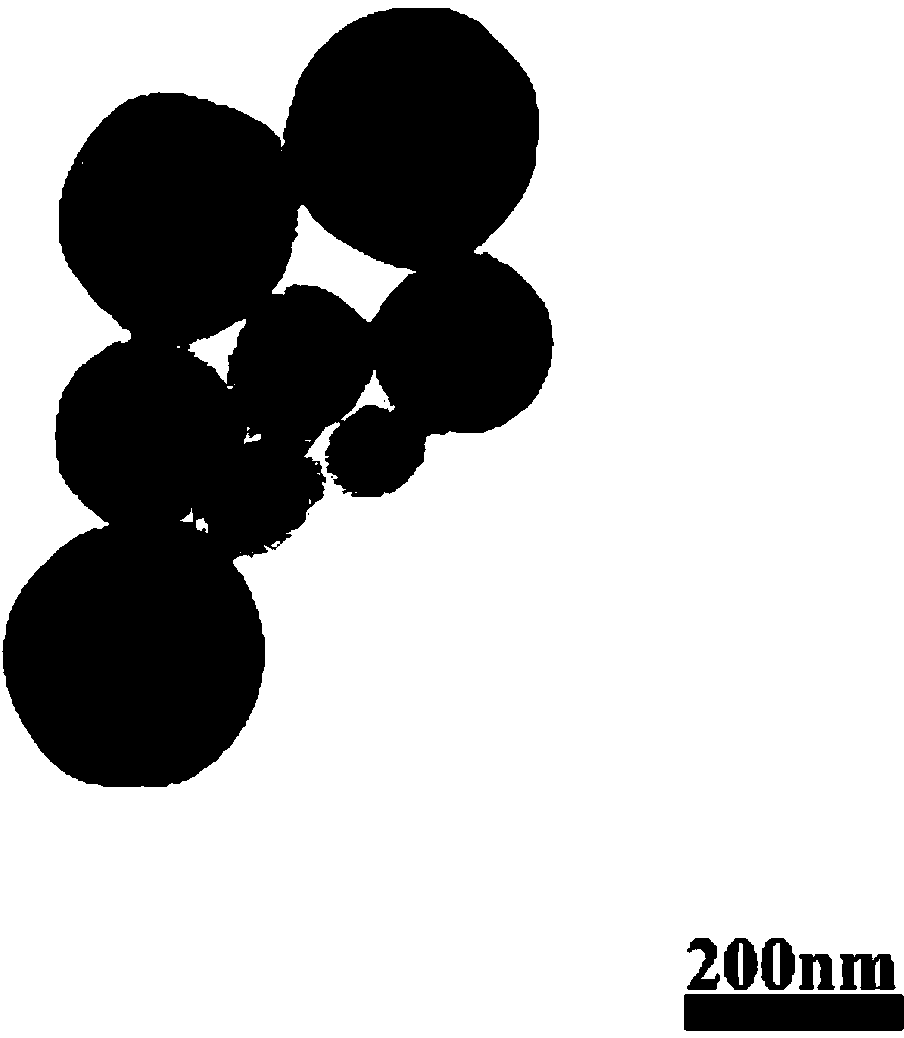
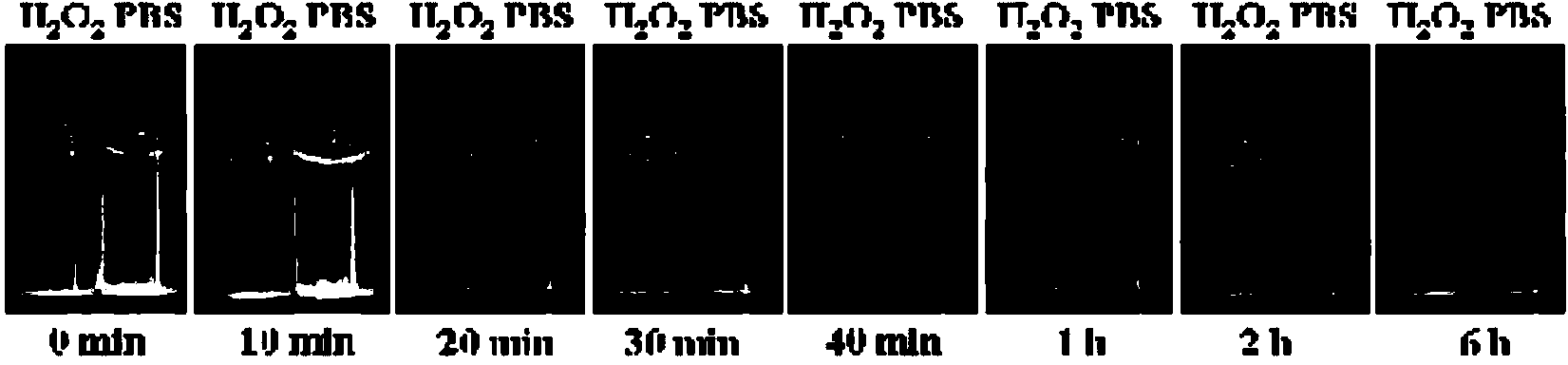
Image
Examples
Embodiment 1
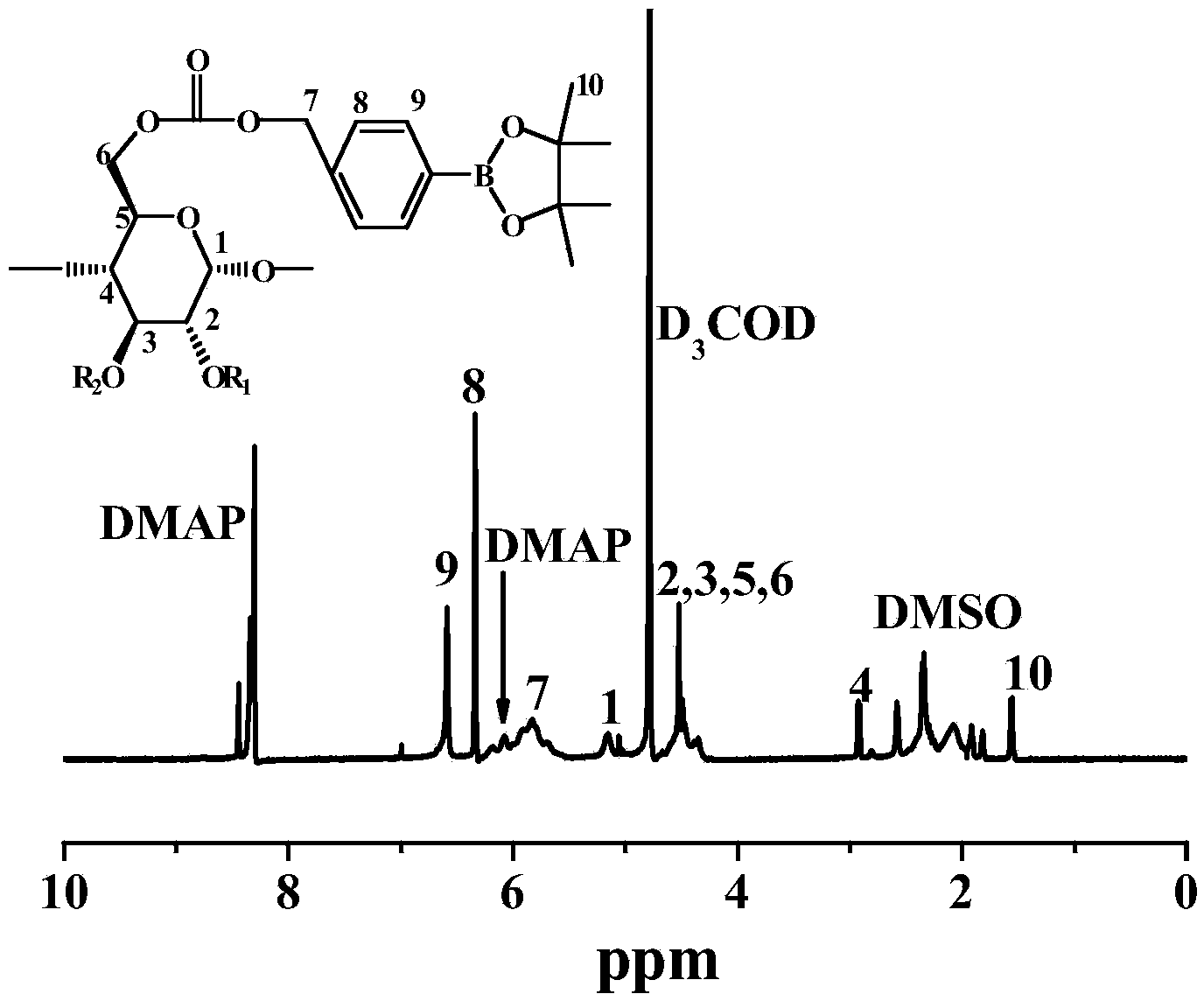
[0038] Under nitrogen protection, 10mmol3-hydroxymethylbenzeneboronic acid and 15mmol pinacol were reacted in 60mL anhydrous tetrahydrofuran to obtain 3-hydroxymethylphenylboronic acid pinacol ester; 5mmol3-hydroxymethylphenylboronic acid pinacol ester was mixed with 10mmol N , N'-carbonyldiimidazole was reacted in 20 mL of anhydrous chloroform to obtain 3-imidazole carbonyloxy-phenylboronic acid pinacol ester; in the presence of 4 mmol 4-dimethylaminopyridine, 0.3 mmol α-cyclodextrin and 4 mmol 3-imidazole Carbonyloxy-phenylboronic acid pinacol ester was reacted in 15 mL of dimethyl sulfoxide at 20° C., and after 8 hours, the cyclodextrin material was obtained by precipitation in water, collected by centrifugation, and dried.
Embodiment 2
[0040] Under nitrogen protection, 10mmol 4-hydroxymethylbenzeneboronic acid and 20mmol pinacol were reacted in 50mL anhydrous dioxane to obtain 4-hydroxymethylphenylboronic acid pinacol ester; 5mmol4-hydroxymethylphenylboronic acid pinacol ester React with 15mmol N,N'-carbonyldiimidazole in 30mL of anhydrous dichloromethane to obtain 4-imidazole carbonyloxy-phenylboronic acid pinacol ester; in the presence of 4mmol N,N'-dicyclohexylcarbodiimide, 0.15mmol of β-cyclodextrin and 4mmol of 4-imidazolecarbonyloxy-phenylboronic acid pinacol ester were reacted in 12mL N,N-dimethylformamide at 30°C, and after 12 hours, they were collected by precipitation in water and centrifugation , and drying to obtain cyclodextrin material.
Embodiment 3
[0042]Under nitrogen protection, 10mmol3-hydroxymethylbenzeneboronic acid and 25mmol pinacol react in 60mL anhydrous acetonitrile to obtain 3-hydroxymethylphenylboronic acid pinacol ester; 8mmol3-hydroxymethylphenylboronic acid pinacol ester and 20mmol N , N'-carbonyldiimidazole was reacted in 25 mL of anhydrous ethyl acetate to obtain 3-imidazole carbonyloxy-phenylboronic acid pinacol ester; in the presence of 4.8 mmol N, N'-diisopropylcarbodiimide, 0.1 One mmol of γ-cyclodextrin reacted with 4.8 mmol of 3-imidazolecarbonyloxy-phenylboronic acid pinacol ester in 10 mL of N,N-dimethylacetamide at 40°C, and after 24 hours, it was collected by precipitation in water and centrifugation , and drying to obtain cyclodextrin material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com