A doxorubicin derivative based on mercapto-betaine modification, nano drug and preparation method thereof
A technology of nano-drugs and derivatives, applied in the field of biomedicine, can solve the problems of adriamycin, such as large toxic and side effects, long blood circulation time, and small toxic and side effects, and achieve fast drug release ability, excellent anti-tumor effect, and reduced toxicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
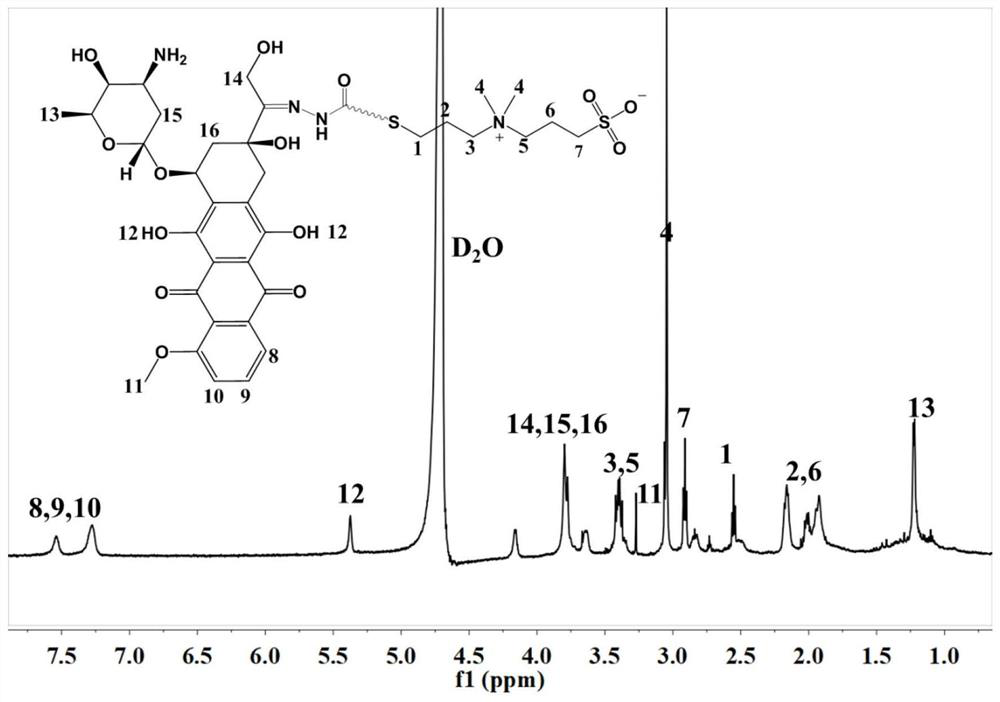
[0061] This embodiment provides a doxorubicin derivative modified by mercapto sulfobetaine, and nanomedicine formed by its self-assembly, mercapto sulfobetaine is selected from 3-((3-mercaptopropyl) dimethyl Ammonium) propane-1-sulfonate, its concrete structure is as follows, and concrete experimental procedure comprises:
[0062]
[0063] 3-((3-Mercaptopropyl)dimethylammonium)propane-1-sulfonate
[0064] (1) According to the molar ratio of mercaptosulfobetaine and 2-methacryloylhydrazide hydrogen bromide to the ratio of 1:2, mix 74.6mg of 2-methacrylohydrazide hydrogen bromide with 50mg of The mercaptolated sulfobetaine was dissolved in 5 mL of methanol solution and stirred for 10 min under nitrogen gas. After removing the nitrogen gas and sealing off the oxygen, it was placed in a 35°C water bath and stirred for 24 h. Rotate the reacted solution to dryness, then add 1mL volume of methanol to dissolve, drop the methanol solution into 9mL acetonitrile, then add 9mL ether, ...
Embodiment 2
[0074] Based on the thiol SB-hyd-DOX prepared in Example 1, this example provides a nanomedicine formed by mixing and self-assembling a mercaptosulfobetaine-modified doxorubicin derivative and paclitaxel. The specific experimental steps include:
[0075] According to the mass ratio of mercapto SB-hyd-DOX and paclitaxel of 1:0.2, mercapto SB-hyd-DOX and paclitaxel were respectively prepared as 5 mg / mL dimethyl sulfoxide solution, and the mercapto SB-hyd-DOX solution was taken separately 83 μL, paclitaxel solution 16.6 μL; the mass concentration of the solution after blending doxorubicin derivatives and paclitaxel is 5 mg / mL, after mixing well, add it dropwise into the phosphate buffer solution with pH 7.4 and stir to obtain thiol SB-hyd-DOX@ PTX self-assembled nanomedicine.
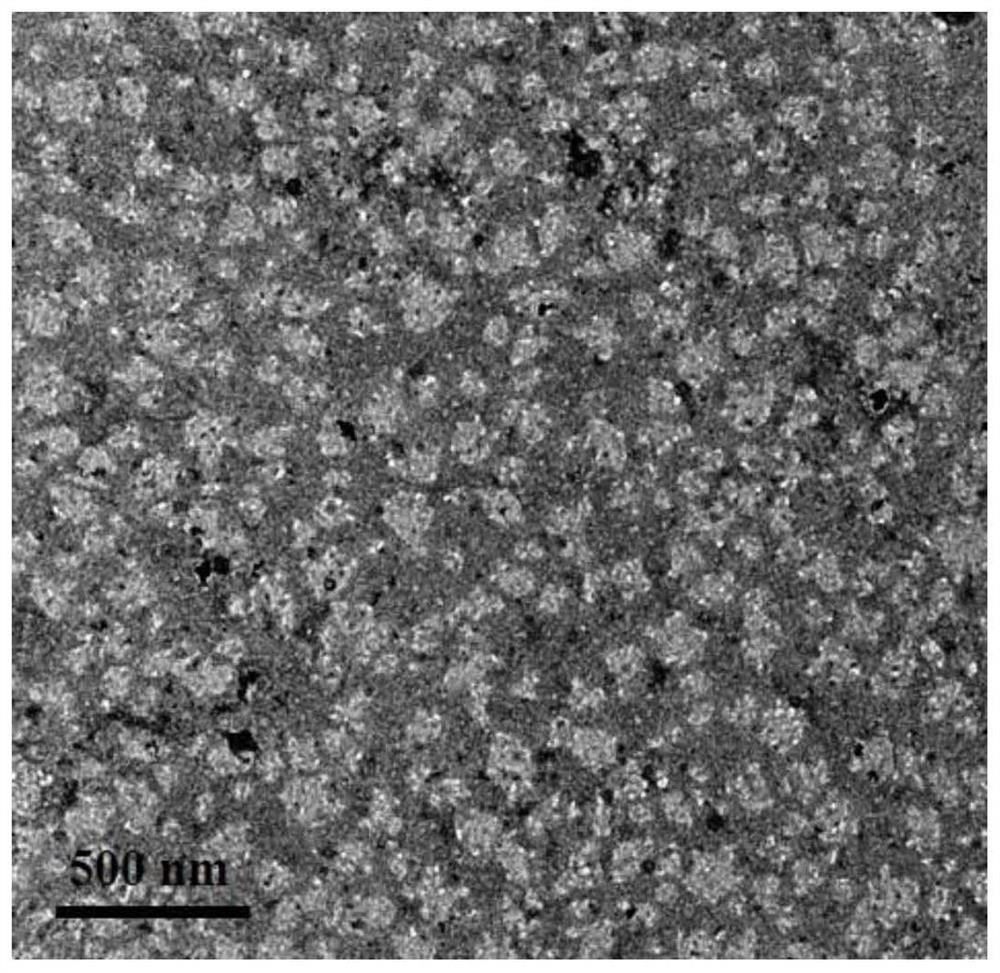
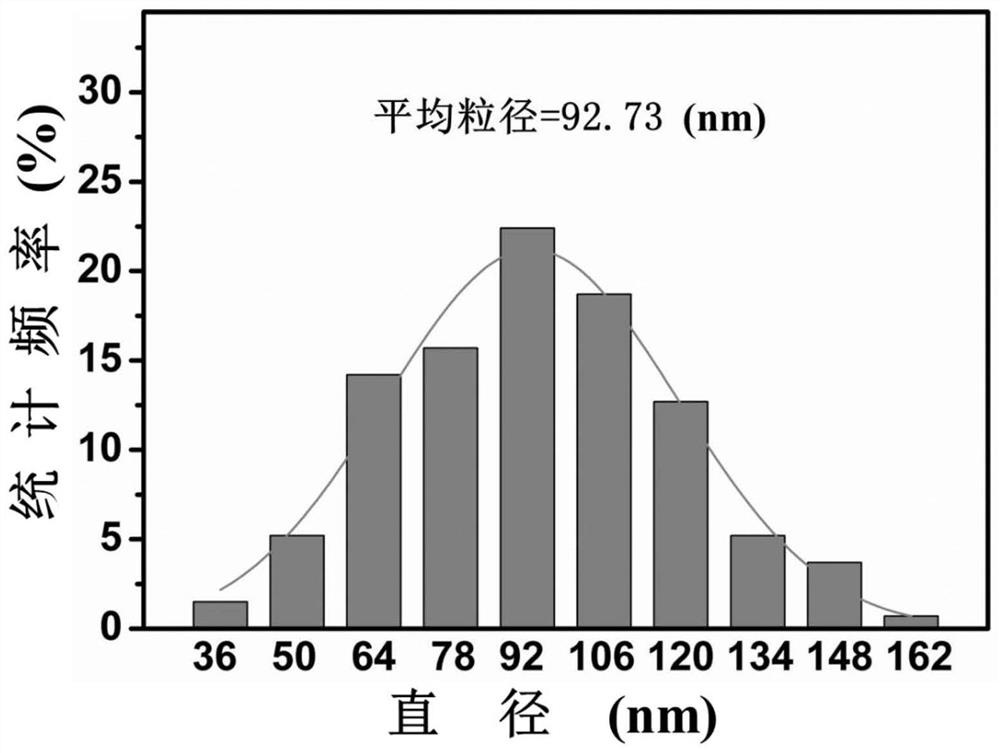
[0076] To characterize the prepared nanomedicine, such as Figure 11 Shown:
[0077] Figure 11 is the hydrodynamic particle size of thiol SB-hyd-DOX@PTX nanomedicine in phosphate buffer solution with p...
Embodiment 3
[0079] This embodiment provides a doxorubicin complex modified by mercaptocarboxybetaine, and nanomedicine formed by its self-assembly. The mercaptocarboxybetaine is selected from 2-((mercaptomethyl)dimethylammonium) ethyl Salt, the specific structure is as follows, and the specific experimental steps include:
[0080]
[0081] 2-((Mercaptomethyl)dimethylammonium)acetate
[0082] (1) Dissolve 50 mg of 2-methacrylohydrazide hydrogen bromide in 41.7 mg of The mercaptolated carboxybetaine was dissolved in 10mL of methanol solution, stirred for 10min with nitrogen gas, and reacted for 6h after removing the nitrogen gas and sealing the oxygen. The reacted solution was evaporated to dryness, and then 1 mL of methanol was added to dissolve it. Add the methanol solution dropwise to 9 mL of acetonitrile, then add 9 mL of ether, centrifuge to remove the lower precipitate, and dry to obtain 2-methacrylohydrazide hydrogen bromide-modified mercaptocarboxybetaine, that is, mercapto CB-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com