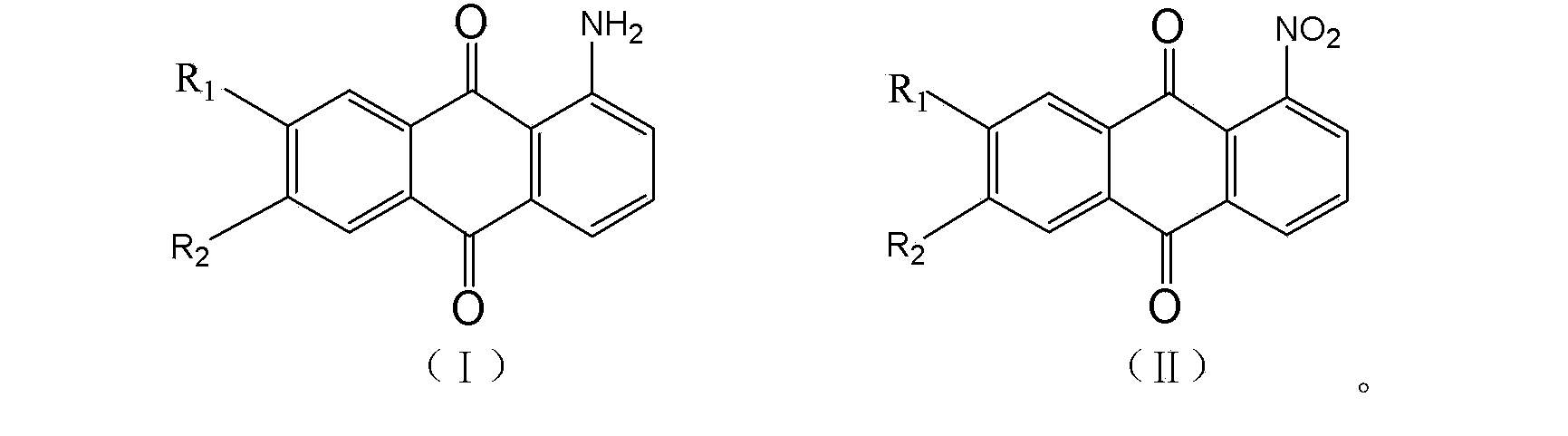
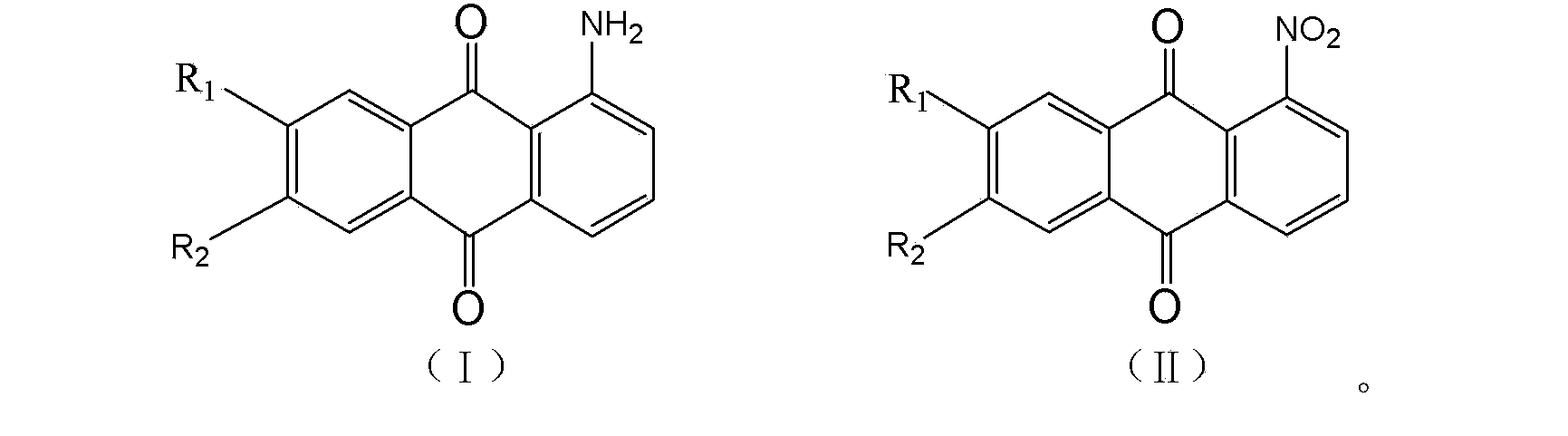
Production method for high-purity 1-aminoanthraquinone
An aminoanthraquinone, production method technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of no industrialization value and high cost, achieve good industrialization value, reduce production costs, equipment Requirements and the effect of low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a 2L electromagnetically stirred hydrogenation pressure reactor, add 120g of 1-nitroanthraquinone with a purity of 99.2%, 1500g of toluene, and 11.0g of Raney Ni catalyst. After replacing the air with inert gas, introduce hydrogen into the system. The hydrogenation reaction is carried out, and the reaction is stopped after the hydrogen consumption reaches the reaction stoichiometric ratio. The reducing solution was heated and filtered to remove the catalyst, and then entered the oxidation tank, fed with oxygen, heated to 105°C and stirred for 1 hour, then cooled to room temperature for crystallization. Filter and crystallize, weigh 95.4g after drying, and check its purity by liquid chromatography. The content of 1-aminoanthraquinone is 99.3%.
Embodiment 2
[0020] In a 2L electromagnetically stirred hydrogenation pressure reactor, add 120g of 1-nitroanthraquinone with a purity of 99.2%, 1200g of xylene, and 10.0g of Raney Ni catalyst, replace the air with an inert gas, and introduce hydrogen into the system. The hydrogenation reaction is carried out at ℃, and the reaction is stopped after the hydrogen consumption reaches the reaction stoichiometric ratio. After the reducing solution is heated and filtered to remove the catalyst, it enters the oxidation tank, is fed with oxygen, is heated to 135°C and stirred for 1 hour, and then cooled to room temperature for crystallization. Filter and crystallize, weigh 97.5g after drying, and check its purity by liquid chromatography. The content of 1-aminoanthraquinone is 99.2%. Apply the mother liquor and catalyst mechanically, add 112g of 1-nitroanthraquinone, repeat the above reaction and post-treatment process (among them, add 3.0g of catalyst for the seventh time of mechanical applicatio...
Embodiment 3
[0022] Raw material, solvent, catalyst consumption and hydrogenation reaction process are all with embodiment 2. Afterwards, the reducing solution was heated and filtered to remove the catalyst and then entered into the oxidation tank, where oxygen and a small amount of ammonia gas were introduced, heated to 135°C and stirred for 1 hour, then cooled to room temperature for crystallization. Filter and crystallize, weigh 98.4g after drying, and check its purity by liquid chromatography. The content of 1-aminoanthraquinone is 99.3%. Apply the mother liquor and catalyst mechanically, add 112g of 1-nitroanthraquinone, repeat the above reaction and post-treatment process (among them, add 3.0g of catalyst for the seventh time of mechanical application), and perform liquid phase analysis on each precipitated crystal obtained from 10 times of mechanical application. Chromatographic detection shows that its 1-aminoanthraquinone content is above 99.1%. The final mother liquor was distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com