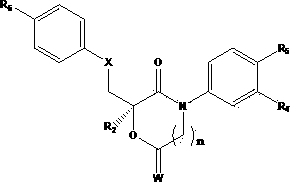
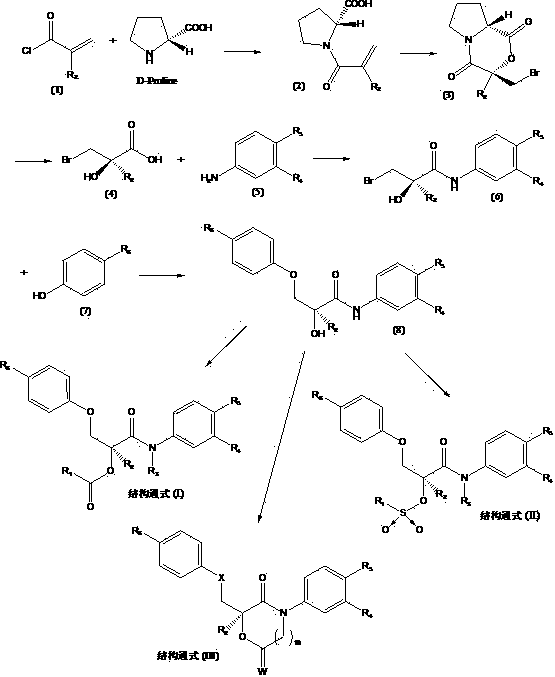
Novel ester group-containing aromatic propionamide compound as well as preparation method and application thereof
A technology for aromatic propionamides and compounds, applied in the field of new ester-containing aromatic propionamide compounds and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
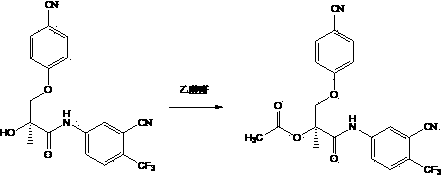
Embodiment 1
[0031] (S)-1-(4-cyano-3-(trifluoromethyl)anilino)-3-(4-cyanophenoxy)-2-methyl-1-oxypropyl-2-ethane Ester, (C 21 h 16 f 3 N 3 o 4 )
[0032]
[0033] Add 2.00 grams of raw materials, 1.05 grams of acetic anhydride, and 20 mL of anhydrous pyridine as a solvent in a 50 mL round bottom flask, reflux, stir, and the reaction time is 2.5 to 3.5 hours. Point the chromatography plate to determine the end point. Thin layer chromatography: ethyl acetate: Hexane=1:1, the product point is relatively single, and there is no raw material point.
[0034] After the reaction was completed, cool and drain to obtain an oil, which was separated and purified by silica gel column chromatography (dichloromethane: ethyl acetate = 98:2~95:5) to obtain a white powder, which was coaxed to dry and weighed. Yield 2.00 g, about 90.0%.
[0035] NMR spectrum: 1 H NMR (500 MHz, DMSO-d 6 ) δ 10.48(s, 1H, NH), 8.29(s, 1H, ArH), 8.17(d, J=8.5Hz, 1H, ArH), 8.11(d, J=8.5Hz, 1H, ArH), 7.77( d, J=8.5Hz, ...
Embodiment 2
[0037] (S)-1-(4-cyano-3-(trifluoromethyl)anilino)-3-(4-cyanophenoxy)-2-methyl-1-oxypropyl-2-benzene Formate, (C 26 h 18 f 3 N 3 o 4 )
[0038]
[0039] Add 5.00 grams of raw materials, 14.53 grams of benzoic anhydride, and 35 mL of anhydrous pyridine as a solvent in a 100 mL round-bottomed flask, reflux, stir, and the reaction time is 9.5 to 10.5 hours. Point the chromatography plate to determine the end point, thin-layer chromatography: ethyl acetate : Hexane=1:1, the product point is relatively single, and there is no raw material point.
[0040] After the reaction was completed, cool and drain to obtain an oily substance, which was separated and purified by silica gel column chromatography (dichloromethane: ethyl acetate=95:5) to obtain a white powder substance, which was coaxed to dryness and weighed to obtain 6.00 grams. The yield is about 96.0%.
[0041] NMR spectrum: 1 H NMR (500 MHz, DMSO-d 6 ) δ 10.59(s, 1H, NH), 8.30(s, 1H, ArH), 8.18(d, J=8.5Hz, 1H, ArH)...
Embodiment 3
[0043] (S)-1-(4-cyano-3-(trifluoromethyl)anilino)-3-(4-cyanophenoxy)-2-methyl-1-oxypropyl-2-methanol Sulfonate, (C 20 h 16 f 3 N 3 o 5 S)
[0044]
[0045] Add 1.00 g of raw materials and 20 mL of anhydrous tetrahydrofuran as a solvent in a 150 mL round-bottomed flask, and drop the temperature to 0°C, add 0.22 g of sodium hydride, stir for 2-3 hours, then add 0.60 g of methanesulfonyl chloride, raise the temperature to room temperature, and stir , the reaction time is 4-5 hours, point the chromatographic plate to determine the end point, thin-layer chromatography: dichloromethane: ethyl acetate = 19:1, the product point is relatively single, and there is no raw material point.
[0046] After the reaction was completed, it was drained to obtain an oil, which was separated and purified by silica gel column chromatography (dichloromethane:ethyl acetate=9 5:5~9:1) to obtain a white powder, which was coaxed to dry and weighed to obtain 1.05 g, the yield is about 85.1%.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com