Polyketide with quaternized side chain and preparation method thereof as well as anion-exchange membrane
A compound and quaternization technology, which is applied in the field of anion exchange membrane, side chain quaternization polyketide and its preparation, can solve the problems of limited application range, achieve good mechanical properties and improve electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Add 1.0mol 2,2′-dihydroxybinaphthalene, 2.2mol potassium carbonate, 2.0mol 4-bromobutyltrimethylammonium bromide and 3.5L dimethyl sulfoxide into a 6.0L reaction flask with nitrogen gas in turn, at 80°C Under mechanical stirring, react for 24h, then add 2.5molNH 4 PF 6 , continue to stir and react at 80°C for 1 h, cool to room temperature, pour into a large amount of water, filter, wash and dry to obtain a solid, the obtained solid is recrystallized by acetonitrile / ethanol with a weight ratio of 95 / 5, filtered, and vacuum dried, Get the anion as PF 6 - The side chain side chain quaternized binaphthyl monomer Q 4 BN (PF 6 - ).
[0087]
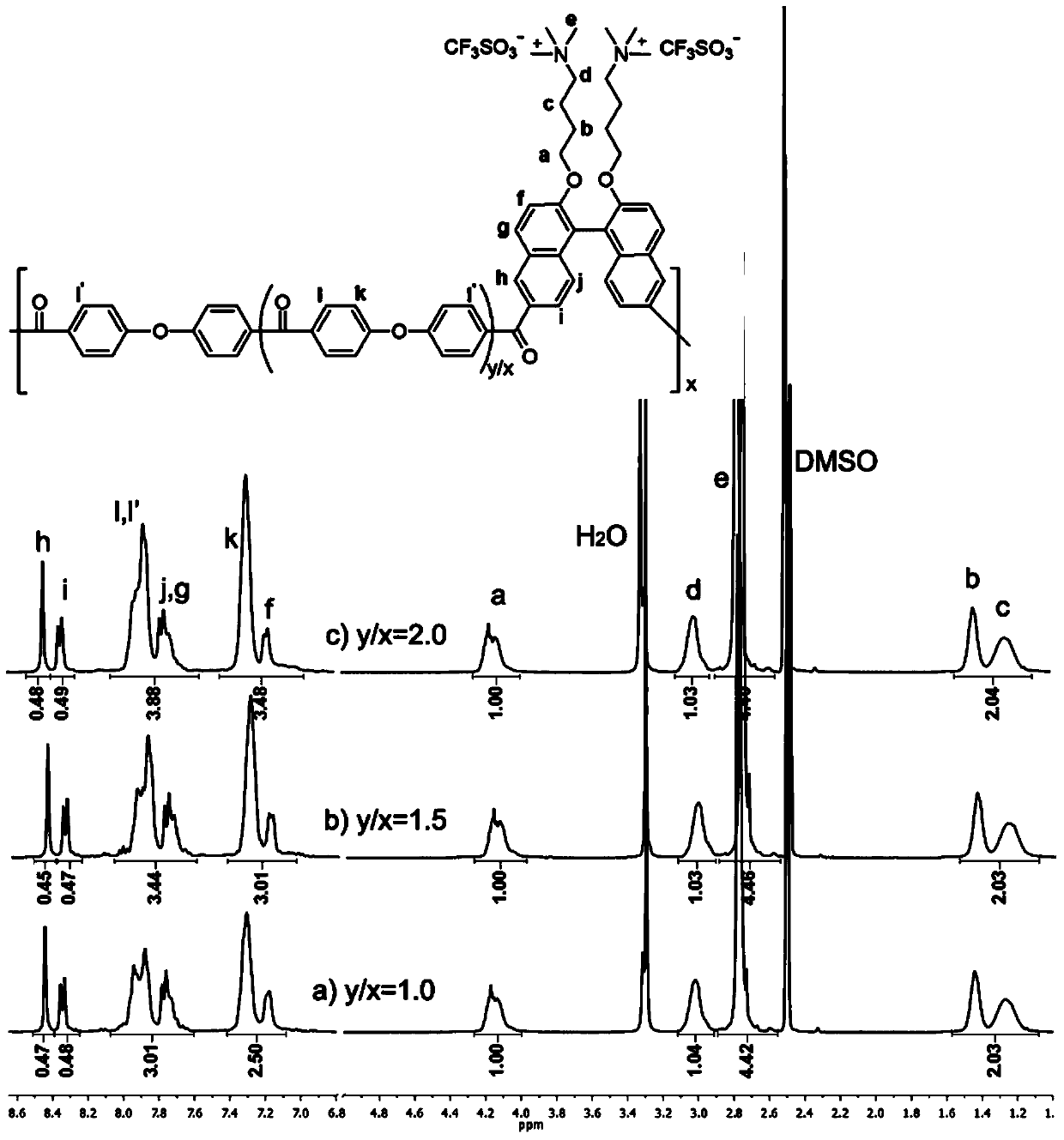
[0088] Utilize nuclear magnetic resonance to Q obtained in embodiment 1 4 BN (PF 6 - ) to detect and get the result: 1 HNMR(400MHz,DMSO)δ8.08(d,J=9.0Hz,2H),7.96(d,J=7.9Hz,2H),7.62(d,J=9.1Hz,2H),7.41–7.22(m, 4H),6.99(d,J=8.5Hz,2H),4.17–3.93(m,4H),3.04–2.85(m,4H),2.72(s,18H),1.47–1.28(m,4H),1.26 –1.05(m,4H).
Embodiment 2
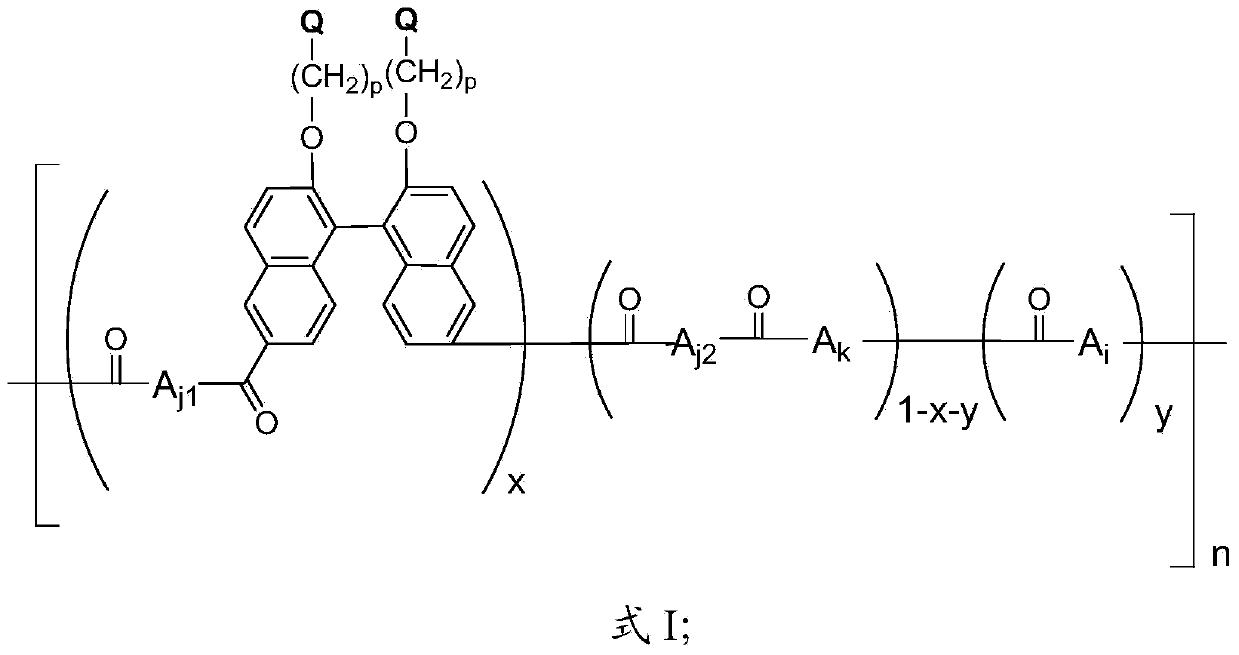
[0090] The Q obtained in 0.01mol embodiment 1 is sequentially 4 BN(PF 6 - ), 1.00mol 4,4′-diphenyl ether dicarboxylic acid, 0.99mol 2,2′-dimethoxybinaphthalene and 4L Eaton’s reagent were added to a nitrogen-gassed reaction flask, stirred and reacted at 60°C for 24 hours, and the reaction solution was heated Pour into a large amount of deionized water to obtain a polymer precipitate, filter and wash repeatedly with deionized water until the filtrate is neutral, and then successively in 1.0mol / L NaNO 3 Soak in the solution at 60°C for 48 hours, and replace NaNO every 12 hours 3 solution; at 1.0mol / L Na 2 CO 3 Soak in the solution at 60°C for 48 hours, and replace Na every 12 hours 2 CO 3 solution; soak in deionized water at 60°C for 48 hours, and replace the deionized water every 12 hours, and then dry to obtain CO 3 2- The side chain quaternized polyketide compound shown in the formula I containing the binaphthyl structure that exists in the form. with CO 3 2- The s...
Embodiment 3
[0094] The side chain quaternized binaphthyl monomer Q that 0.01mol embodiment 1 obtains successively 4 BN(PF 6 - ), 1.00mol 4,4′-diphenyl ether dicarboxylic acid, 0.99mol 2,2′-dimethoxybinaphthalene and 4L Eaton’s reagent were added to a nitrogen-gassed reaction flask, stirred and reacted at 100°C for 4h, and the reaction solution was heated Pour a large amount of deionized water to obtain polymer precipitation, filter and wash repeatedly with deionized water until the filtrate is neutral, soak the obtained polymer with 0.1mol / L dilute NaOH solution at room temperature for 24 hours, and then repeatedly wash with deionized water until it is washed Liquid is neutral. Subsequent ion exchange treatment was the same as in Example 2.
[0095] in CF 3 SO 3 - Strong carbonyl absorption peaks and almost no carboxyl absorption peaks were observed in the infrared spectrum of the side chain quaternized polyketones, indicating the occurrence of polyacylation; H NMR spectra confirmed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com