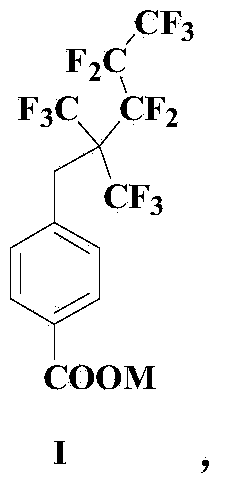
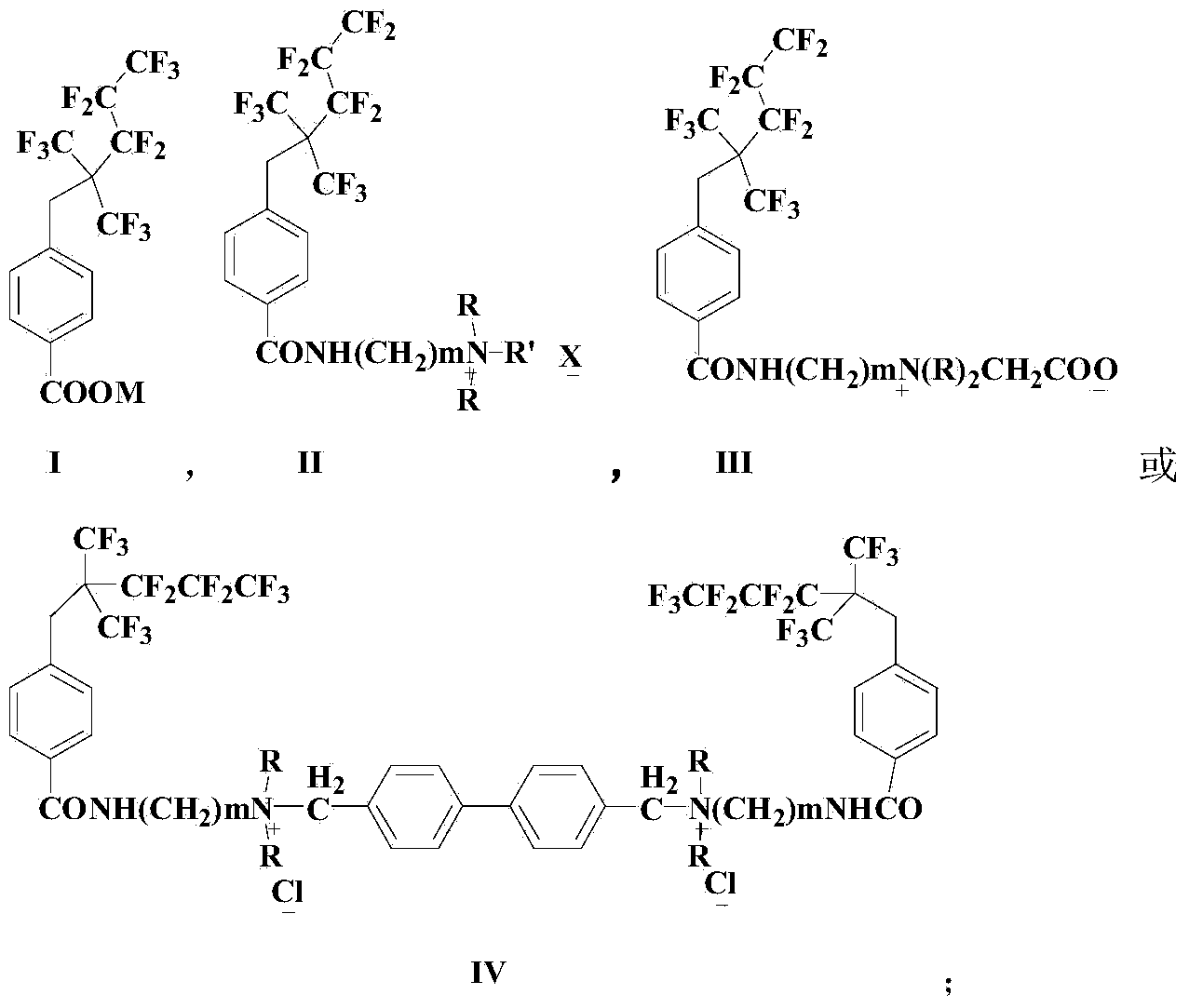Fluorocarbon surface active agent and preparation method thereof
A technology of fluorocarbon surfactants and active agents, which is applied in the field of fluorocarbon surfactants and its preparation, and can solve the problems of low surface activity and application limitations of branched products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
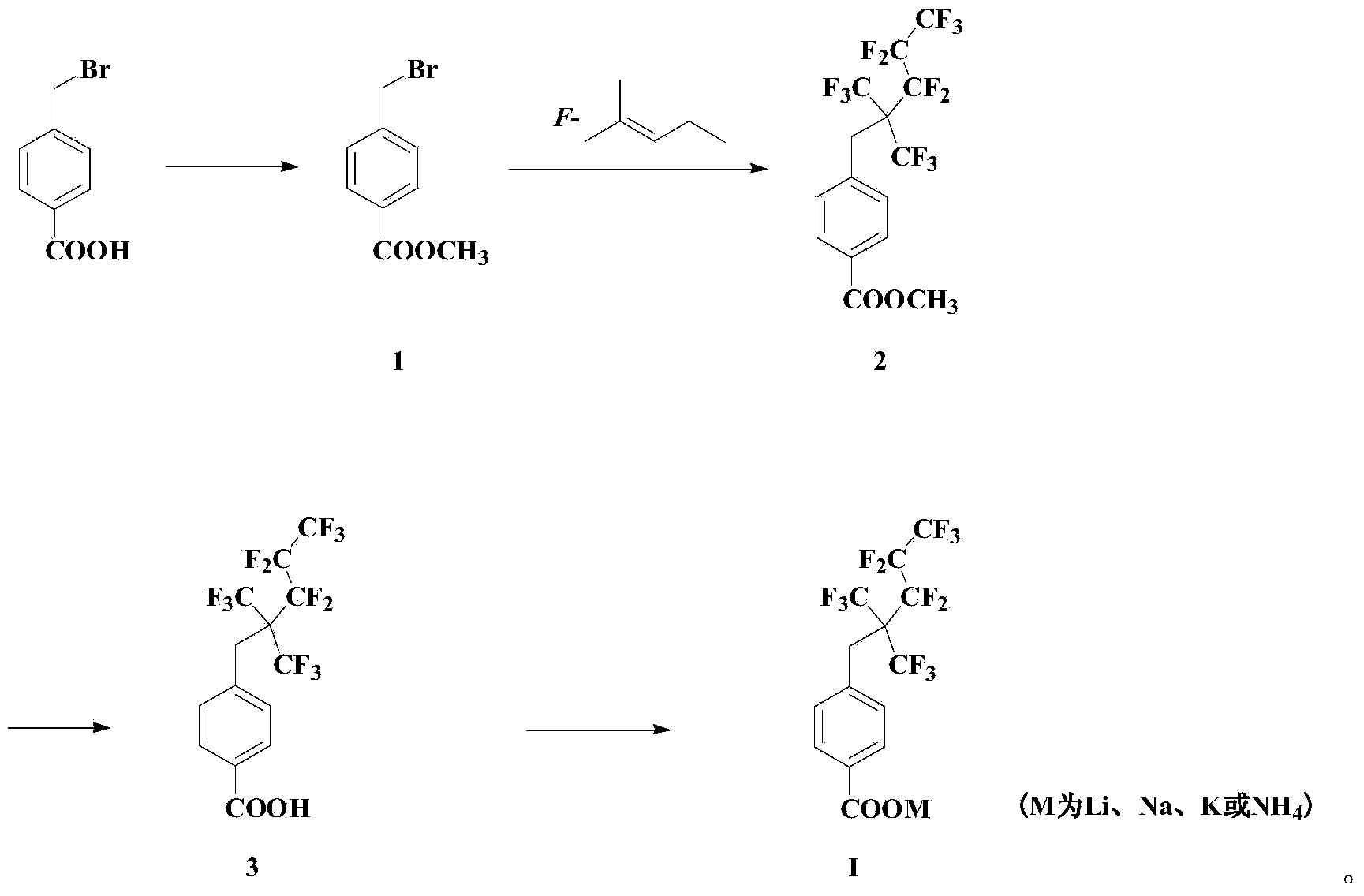
[0070] Embodiment 1: the synthesis of fluorine-containing intermediate organic acid compound 3
[0071]
[0072] 2.14g of p-bromomethylbenzoic acid, 0.61g of DMAP and 4.12g of DCC were sequentially added into a 100mL jacketed reaction tube. After three times of ventilation, 50mL of anhydrous dichloromethane was added in an ice-water bath, and stirred for a while. Excess methanol was added to the system, and the system was moved to room temperature for reaction after the drop was completed, and the reaction was monitored by TLC. After 5 hours the reaction was complete. System filtration, filtrate successively with KHSO 4 solution, deionized water and saturated brine. The organic phase was dried over anhydrous sodium sulfate, filtered, and the solvent was removed. Finally, the mixture was passed through a silica gel column with an eluent of EA:PE=1:20 to obtain 1.942g of compound 1.
[0073] 1 H NMR (CDCl 3 ,300MHz): δ(ppm)3.92(s,CH 3 ,3H),4.50(s,CH 2 ,2H),7.44-7.48(d...
Embodiment 2
[0079]Example 2: Synthesis of anionic fluorocarbon surfactants containing branched fluorocarbon chains
[0080]
[0081] 3.6 g of compound 3 was dissolved in 20 mL of tetrahydrofuran, and lithium hydroxide aqueous solution was slowly added dropwise until the system pH=7-8. After dropping, the mixed system was spin-dried to obtain a viscous solid. The solid was washed and filtered with acetone, the filter cake was dissolved with methanol, and the solvent was removed to obtain 3.1 g of white powdery anionic fluorocarbon surfactant.
[0082] 1 H NMR (CD 3 OD,300MHz):δ(ppm)3.71(s,CH 2 ,2H),7.30-7.37(d,J=8.4Hz,Ar-H,2H),7.86-7.93(d,J=8.4Hz,Ar-H,2H); 19 F NMR (CD 3 OD,282MHz,):δ(ppm)-63.68~-63.90(m,6F),-81.98~-82.14(t,3F),-106.90~-107.20(m,2F),-124.05~-124.40(m ,2F). 13 C NMR (CD 3 OD,100MHz,):δ(ppm)33.32,110~127,130.12,132.18,134.28,139.15,174.80;LRMS(EI):454,437.Anal.Calcd for C 14 h 6 f 13 LiO 2 :Li,1.51.Found:Li,1.48.
Embodiment 3
[0083] Example 3: Synthesis of quaternary ammonium salt cationic fluorocarbon surfactants containing branched fluorocarbon chains
[0084]
[0085] Add 5g of compound 3, 0.675g of DMAP and 4.2g of EDCI in sequence in a 100mL jacketed reaction tube,
[0086] After pumping and exchanging air three times, add 50 mL of dichloromethane under an ice-water bath, and stir for a while. Add excess N,N-dimethylethylenediamine to the system, and after the drop is complete, the system is moved to room temperature for reaction. The reaction was monitored by TLC, and the reaction was completed after 5 hours.
[0087] The system was washed successively with deionized water and saturated brine. The organic phase was dried over anhydrous sodium sulfate, filtered, and the solvent was removed. Finally, the mixture was passed through a silica gel column with dichloromethane:methanol:triethylamine=20:1:a small amount of eluent to obtain 5.126g of compound 7.
[0088] 1 H NMR (DMSO-d 6 ,300...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com