L-ornithine glutamic acid double salt and its preparation method and application
A technology of ornithine glutamic acid and ornithine, which is applied in the fields of medicinal chemistry, biochemistry and medicine, can solve the problem of no L-ornithine glutamic acid double salt crystals, etc., to improve production efficiency and equipment utilization High efficiency, good crystallization effect, and perfect crystal shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
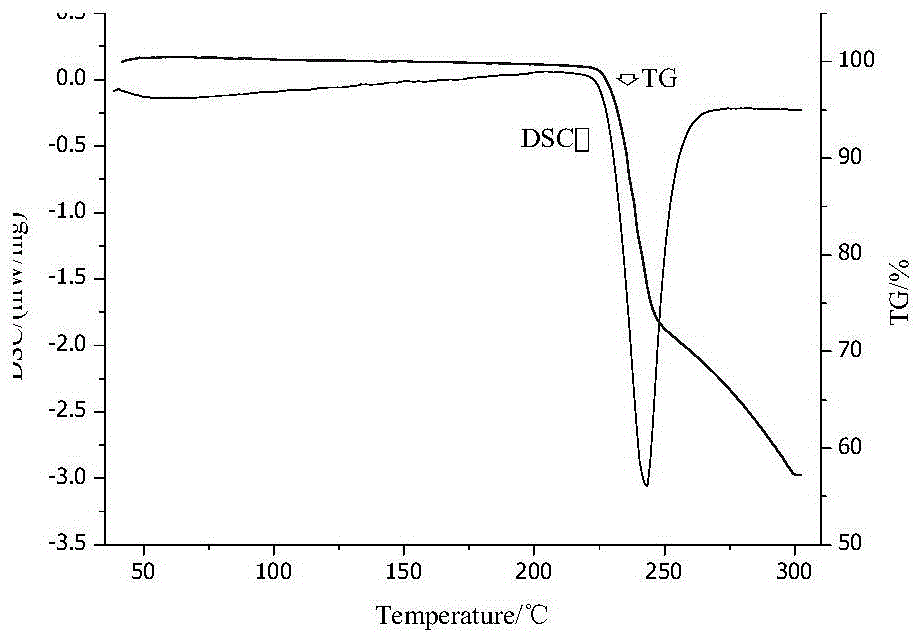
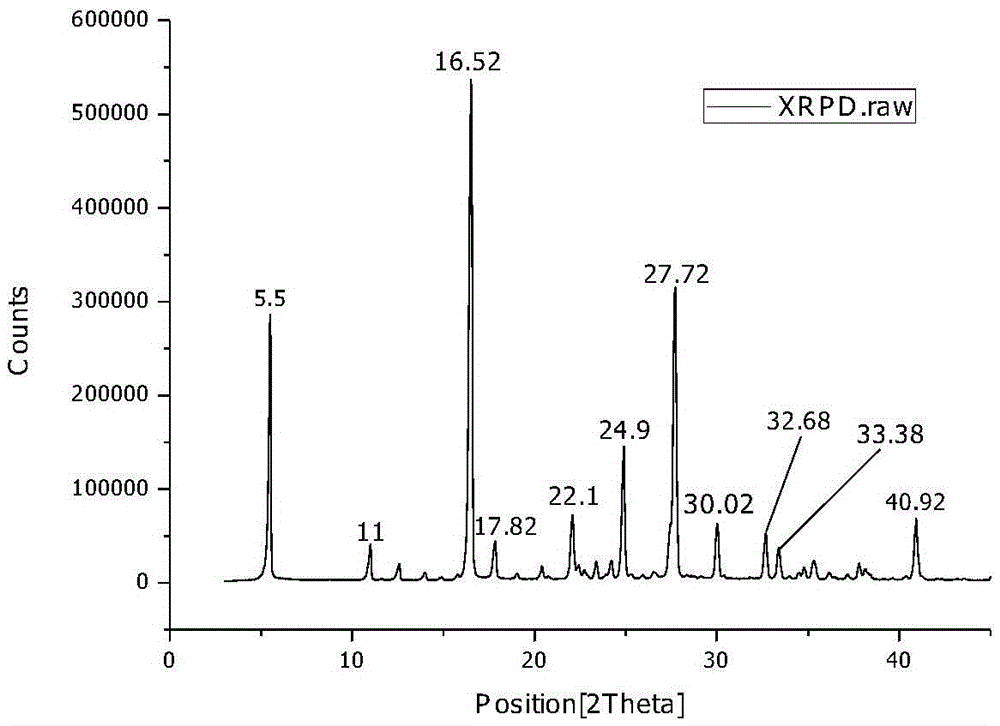
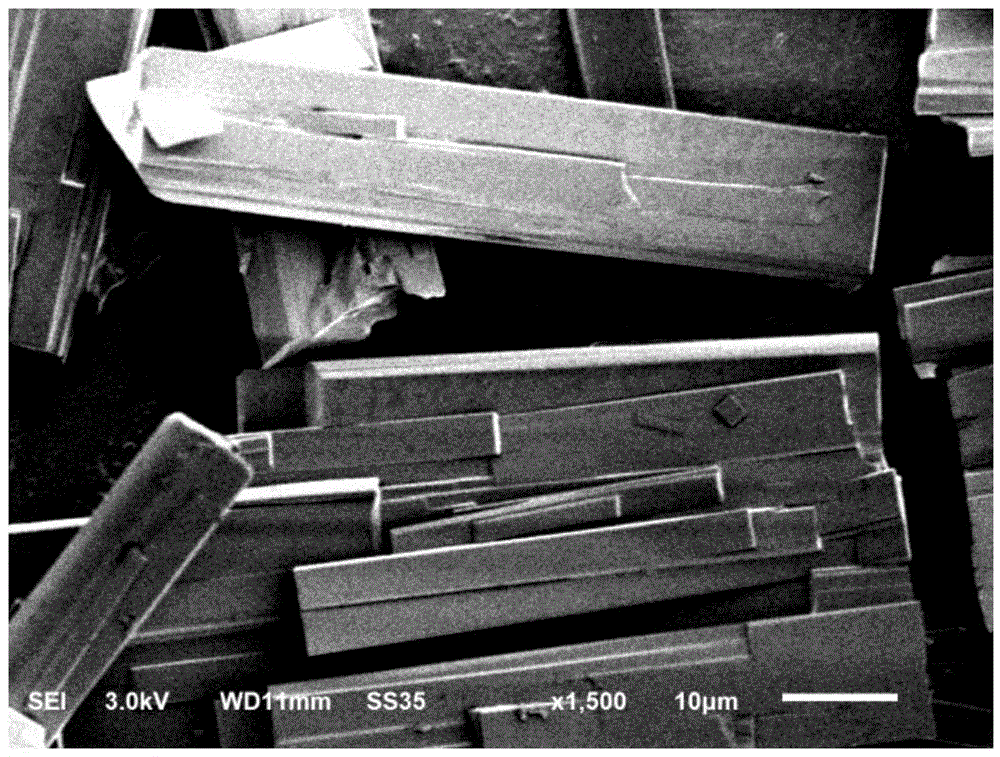
[0041] First, the L-ornithine fermentation liquid (such as the fermentation liquid obtained according to the method of Example 1 or 2 in the document CN101955901A) is pretreated by membrane filtration, continuous separation, decolorization and other processes to obtain a free L-ornithine aqueous solution , and concentrate it to a concentration of 48g / L, take 100ml and pour it into a reaction crystallizer (such as Figure 5 shown, the same below), then put 5.31g of glutamic acid powder into it, stir at 25°C for 25 minutes to complete the reaction, add 0.1g of L-ornithine glutamic acid double salt seed crystal, stir for 30 minutes to grow the crystal, drop 150ml ethanol. Then lower the temperature to 10°C and keep the temperature constant for 0.5h. The obtained suspension was suction-filtered, washed, and dried for 5 hours at 20° C. under a vacuum of 0.08 MPa to obtain white columnar crystals of L-ornithine glutamic acid double salt. Its purity was determined to be 99.2%, and ...
Embodiment 2
[0043] L-ornithine hydrochloride was dechlorinated to obtain free L-ornithine aqueous solution, concentrated under reduced pressure until the concentration of free L-ornithine was 46g / L, took 100ml and poured it into a reaction crystallizer, and then put 5.14g into it Glutamic acid powder, stirred at 22°C for 30 minutes to complete the reaction, added 0.08 g of L-ornithine glutamic acid double salt seed crystal, stirred for 45 minutes, and added dropwise 200 ml of methanol. Then lower the temperature to 8°C and keep the temperature constant for 0.5h. The obtained suspension was suction-filtered, washed, and dried at 30° C. under a vacuum of 0.08 MPa for 4 hours to obtain white columnar crystals of L-ornithine glutamic acid double salt. Its purity was determined to be 99.3%, and the yield was 95.6%.
Embodiment 3
[0045] First, pretreat the L-ornithine fermentation liquid (such as the fermentation liquid obtained according to the method of Example 1 or 2 in the document CN101955901A) (the pretreatment method is the same as in Example 1) to obtain a free L-ornithine aqueous solution, and Concentrate it to a concentration of 46g / L, take 150ml and pour it into a reaction crystallizer, then put 7.1g of glutamic acid powder into it, stir at a constant temperature of 20°C for 20min to complete the reaction, add L-ornithine glutamic acid double salt crystal Seed 0.2g, stir and grow the crystal for 45min, and add 300ml of acetone dropwise. Then lower the temperature to 10°C and keep the temperature constant for 0.5h. The obtained suspension was suction-filtered, washed, and dried for 3 hours at 20° C. under a vacuum of 0.08 MPa to obtain white columnar crystals of L-ornithine glutamic acid double salt. Its purity was determined to be 99.4%, and the yield was 94.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com