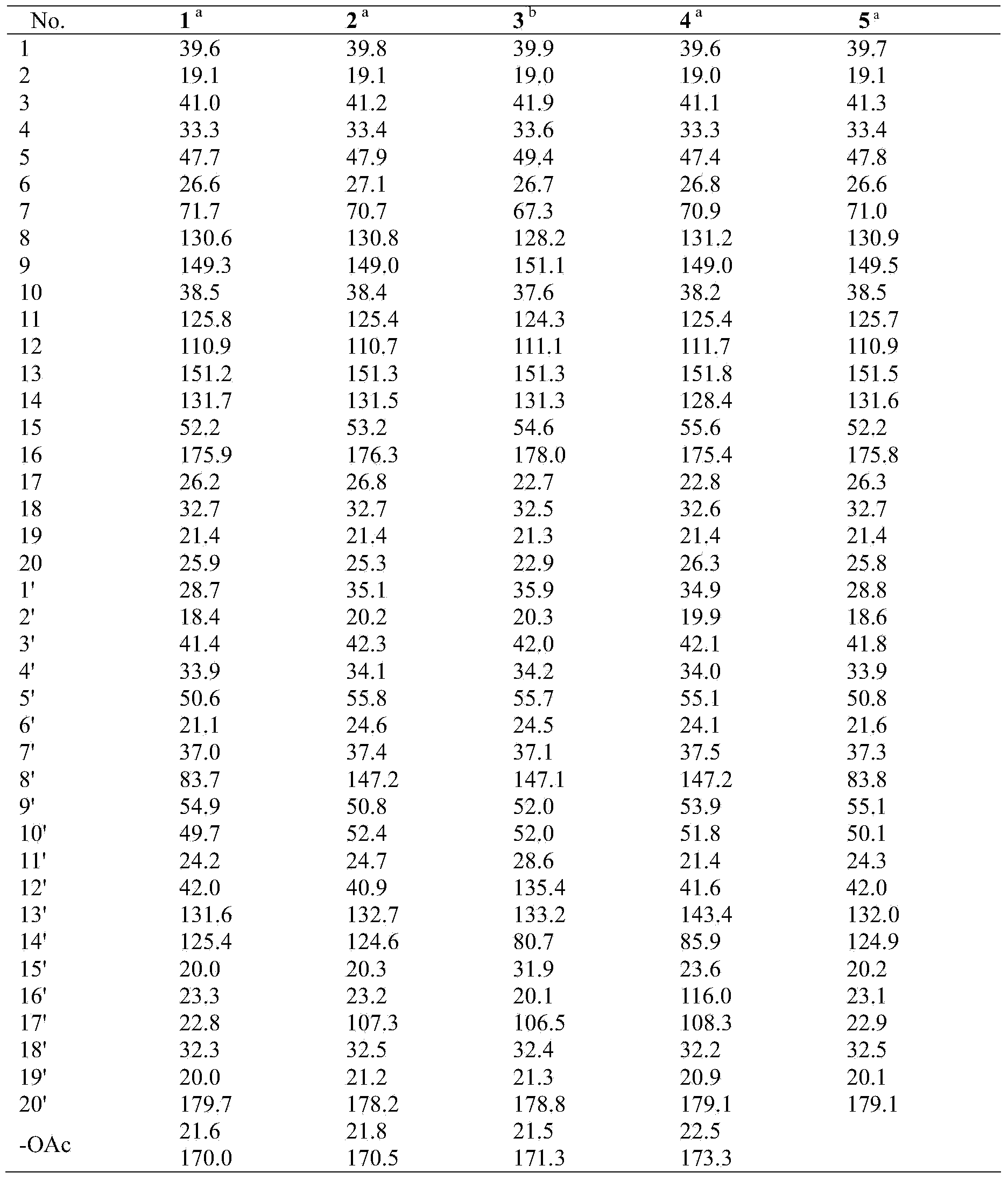Diterpene dimer compounds and pharmaceutical compositions and preparation method and application thereof
A technology of diterpene dimer and compound, applied in the field of natural medicinal chemistry, can solve the problems of few research reports and the like, and achieve the effect of good anti-tumor activity and good anti-parasitic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
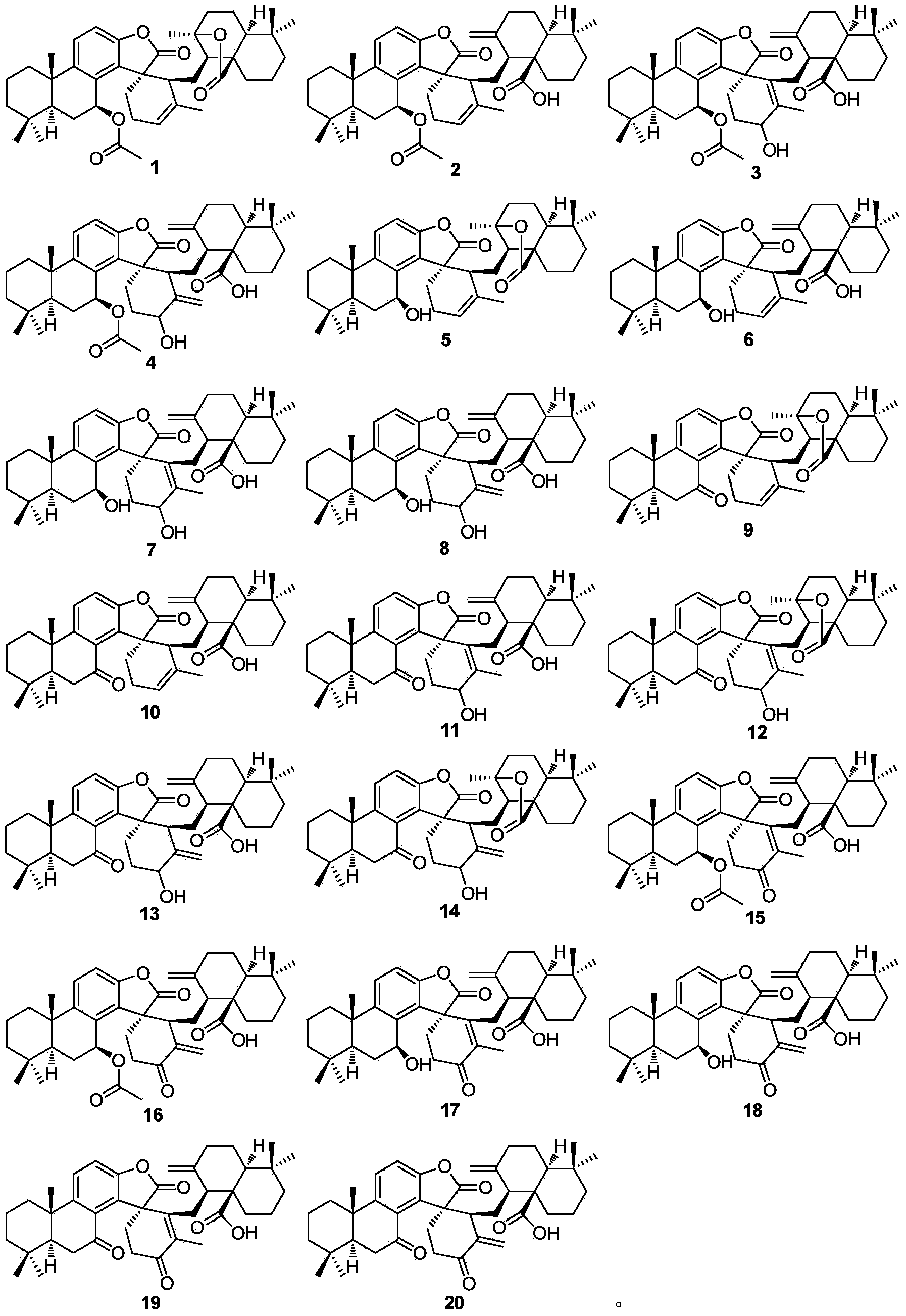
[0029] Extraction, separation and purification of compound 1-20 of the present invention:
[0030] The rhizomes (5.5 kg) of the genus Camellia were dried in the shade, crushed to 30 mesh, extracted three times with 70% acetone at room temperature, 25 L each time, 24 h, the extracts were combined, and the extracts were concentrated under reduced pressure to obtain Suspend the extract with an appropriate amount of water, and then distribute it several times with ethyl acetate to obtain an ethyl acetate extract (115 g). The extract is dissolved in an appropriate amount of chloroform / acetone and mixed with silica gel 80-100 mesh, and then mixed with 1.2 kg of silica gel 200-300 mesh for column chromatography and sectioning, and gradient elution with chloroform / acetone (1 : 0-0 : 1) to obtain 8 main fractions, the chloroform fraction and the 9: 1 chlorine / acetone fraction Silica gel column chromatography was carried out, and 200:1-2:1 petroleum ether / ethyl acetate was used for grad...
Embodiment 2
[0032] Physical and Spectroscopic Data of Compounds 1-20 of the Invention:
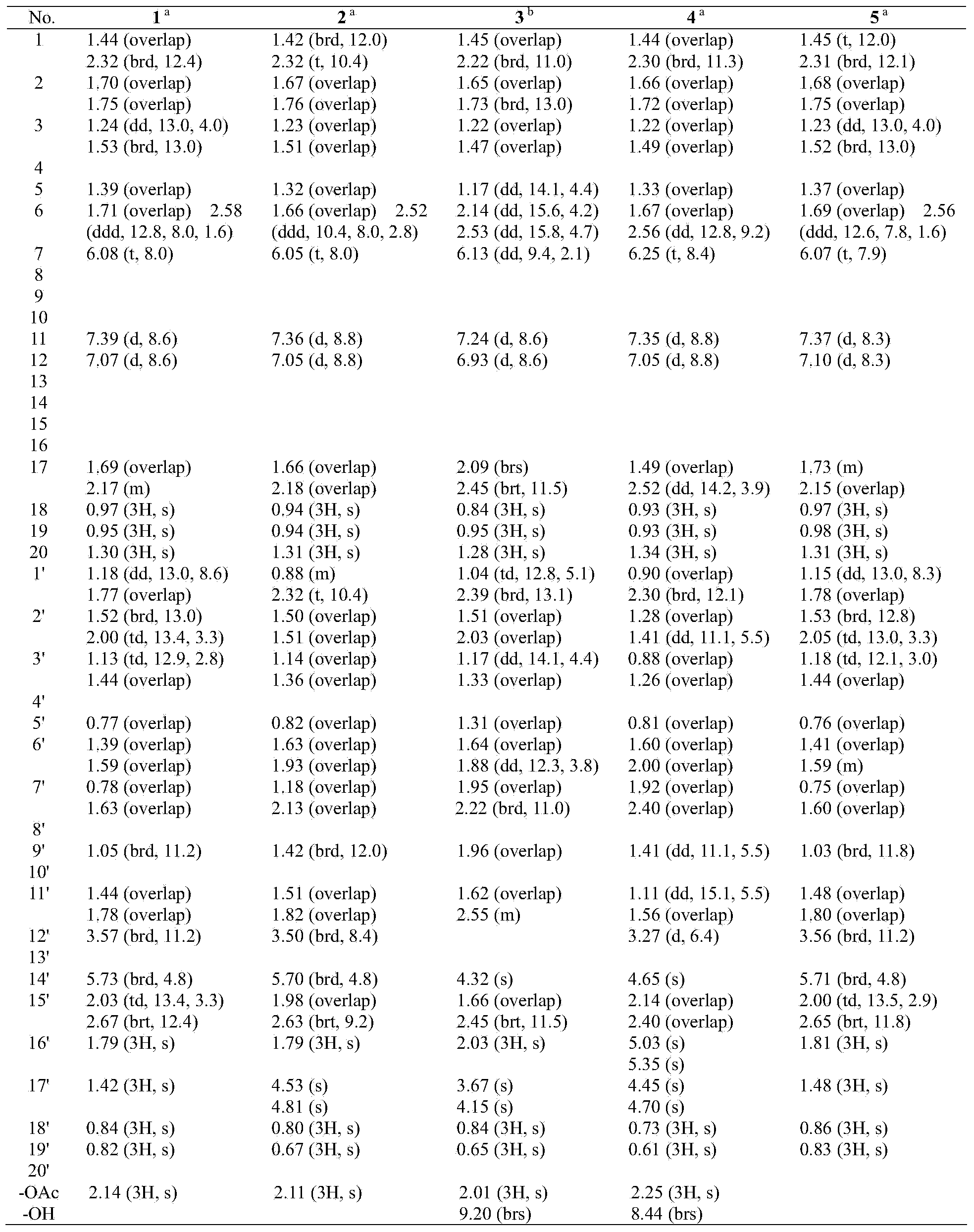
[0033] Compound 1: colorless crystals (acetone), UV (MeOH) λ max (logε) 289.4(3.45), 209.6 (4.63) nm; IR (KBr) v max 3020, 2928, 2868, 1800, 1762, 1728, 1634, 1610, 1586, 1458, 1373, 1278, 1218, 1132, 1027, 943, 823, 756 cm -1 ; EIMS m / z 656 [M] + (5), 596 (25), 294 (44), 85 (75), 83 (100), 69 (40); 1 H and 13 See Table 1 and Table 2 for C NMR data.
[0034] Compound 2: colorless crystals (acetone), UV (MeOH) λ max (logε) 285.6(3.45), 208.6 (4.58) nm; IR (KBr) v max cm -1 ; ESIMS m / z 1336 [2M + Na + H] + , 680 [M + Na + H] + , 619 [M + Na – HOAc] + , 551 [M-HOAc-COOH] + ; 1 H and13 See Table 1 and Table 2 for C NMR data.
[0035] Compound 3: colorless crystals (acetone), UV (MeOH) λ max (logε) 284.1(3.73), 204.0 (4.90) nm; IR (KBr) v max 3421, 3081, 2930, 2869, 1800, 1737, 1716, 1647, 1466, 1368, 1231, 1125, 1045, 945, 819, 756 cm -1 ; EIMS m / z 672 [M] + (4), 671 (8), 62...
Embodiment 3
[0085] Cytotoxic activity detection of compounds of the present invention:
[0086] The cytotoxicity of the compound of the present invention to human gastric cancer cell line (SGC-7901), liver cancer cell line (SMMC-7721) and human erythrocytic leukemia cell line (K-562) was determined by MTT method. In the experiment, a negative control group (water), a DMSO solvent control group, a positive control group (mitomycin C) and different concentrations of test samples were set up, and each concentration was set in 2 parallels. Cells in the logarithmic growth phase were collected, counted with a hemocytometer, and inoculated in a 96-well flat-bottomed cell culture plate according to the amount of 4500 cancer cells per well, placed in 5% CO 2 , Humidity above 90%, cultured in 37 ℃ incubator. After 24 hours, take out and add a certain amount of sample to be tested, continue to cultivate for 3 days, take out and place under a microscope to observe the cell morphology of each well, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com