Method for extracting high-density lipoprotein and separating and purifying apolipoprotein apoA-I from human plasma
A high-density lipoprotein, separation and purification technology, applied in the field of separation and purification of high-purity apolipoprotein apoA-I, can solve the problems of protein activity loss, denaturation, HDL protein oxidation, etc. The effect of purity and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Extraction of HDL by ultracentrifugation
[0041] Use sodium bromide (analytical pure) to adjust the density (D1) to 1.09g / ml in 500ml of normal human plasma, ultracentrifuge in a Hitachi centrifuge at 50000rpm, 8°C, 24hr; discard the yellow component at the top of the centrifuge tube, and collect the dark yellow component at the bottom of the tube Part I, adjust the density with sodium bromide (D 2 ) to 1.21g / ml, centrifuge for 24hr under the same conditions, collect about 120ml of the light yellow component II in the upper third of the centrifuge tube, then dilute and mix with 2 times the volume of sodium bromide solution with a density of 1.21, and centrifuge at Hitachi Machine ultracentrifugation at 50,000 rpm, 8°C, 24 hr, and repeat once under the same conditions, to obtain about 20 ml of pure HDL as the light yellow component in the top layer.
Embodiment 2
[0042] Embodiment 2: Improve Scanu method degreasing
[0043]Concentrate 20ml of the HDL solution obtained by centrifugation in Example 1 to a higher concentration by ultrafiltration, and add dropwise to 700ml of -21°C pre-cooled absolute ethanol / anhydrous ether (V / V ) 3:2, stir at -21°C for 6hr, let stand overnight, centrifuge at 5000rpm at -4°C for 10min, remove the supernatant, leave the precipitate, and redissolve the precipitate in absolute ethanol / anhydrous ethyl alcohol (V / V ) 3:2, repeat the above steps once, and then redissolve the precipitate in 50ml of pre-cooled anhydrous ether, centrifuge to remove the supernatant, evaporate the precipitate in a ventilated place, and store at -21°C to obtain the defatted apoHDL.
Embodiment 3
[0044] Embodiment 3: the molecular sieve chromatography purification of apoHDL
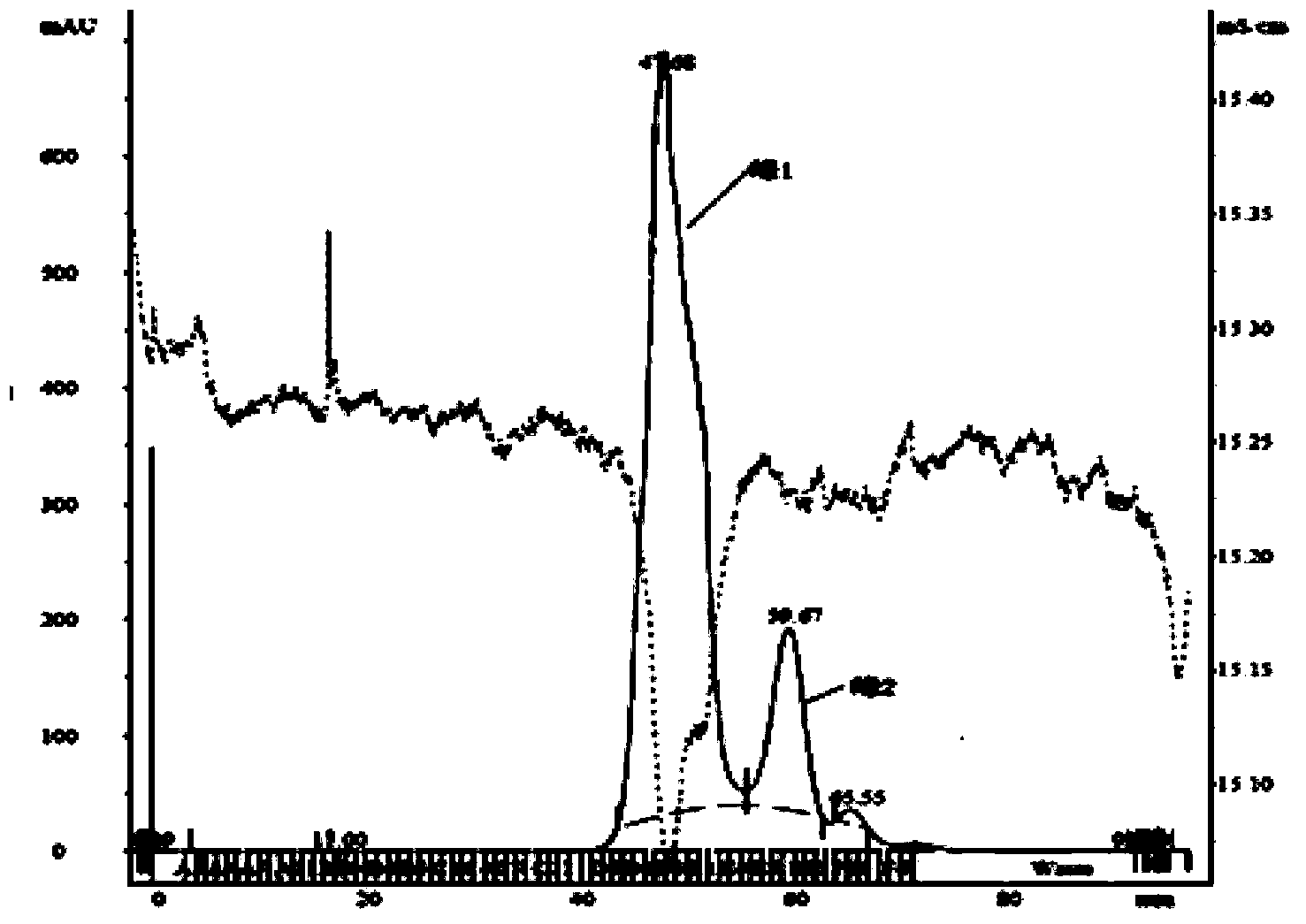
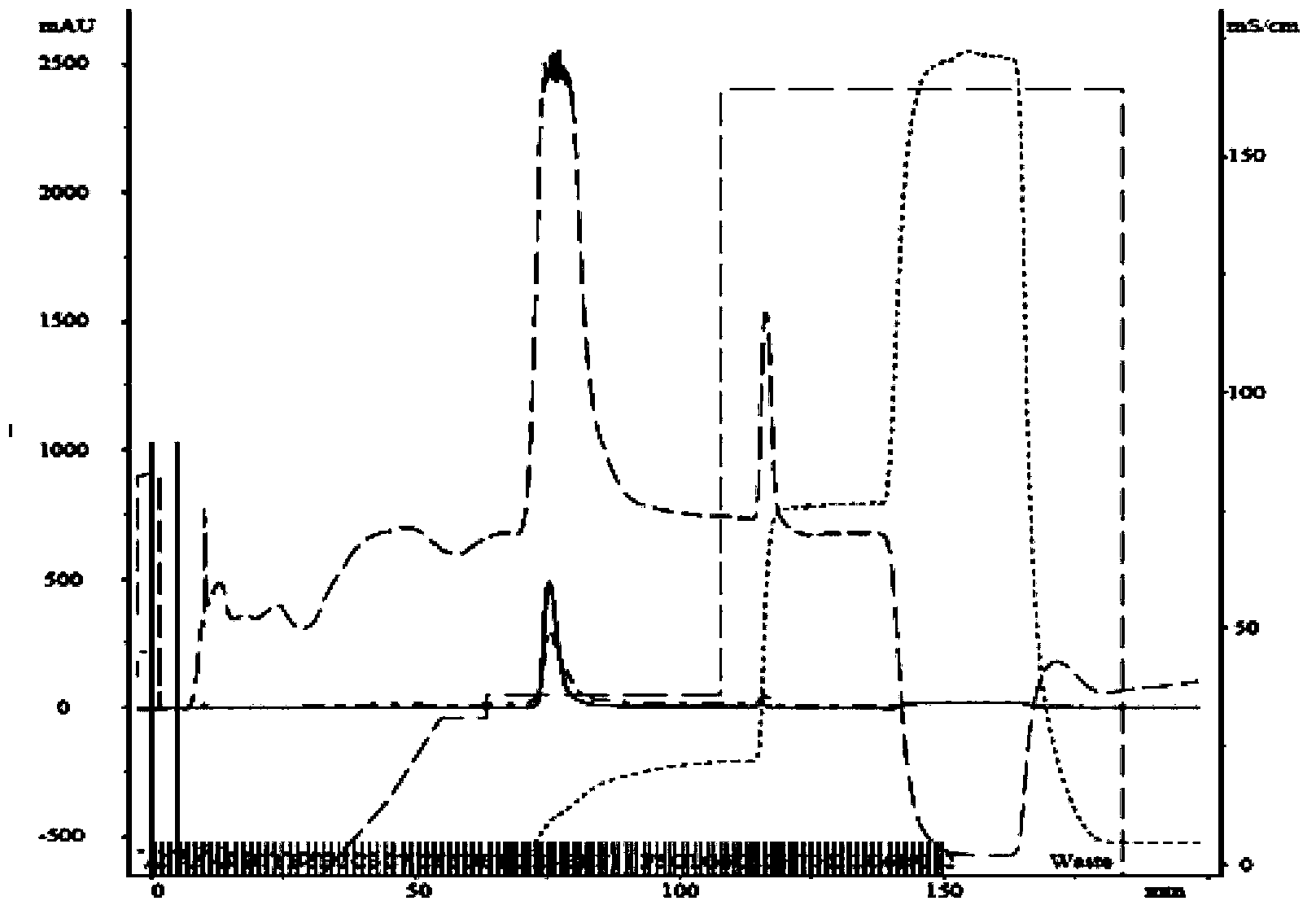
[0045] About 500 mg of apoHDL after degreasing in Example 2 was redissolved in 10 ml of 25 mM Tris-HCl buffer (containing 0.1% EDTA, 0.1% NaN 3 , 150mM NaCl, 6M urea), pH 8.0, so that the final concentration of apoHDL reaches 50mg / 3ml, 0.22μm filter before loading: using GE AKTA TM Fast protein chromatography purifier100 system, Superdex G75 molecular sieve chromatography prepacked column, elution flow rate is 1ml / min, collected by absorption peak, chromatogram and electrophoresis spectrum see figure 1 , 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com