Sulfur-containing substituted two-dimensional conjugated polymer as well as preparation method and application thereof
A two-dimensional conjugated, polymer technology, used in semiconductor/solid-state device manufacturing, electrical components, circuits, etc., can solve problems such as restricting the practical process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
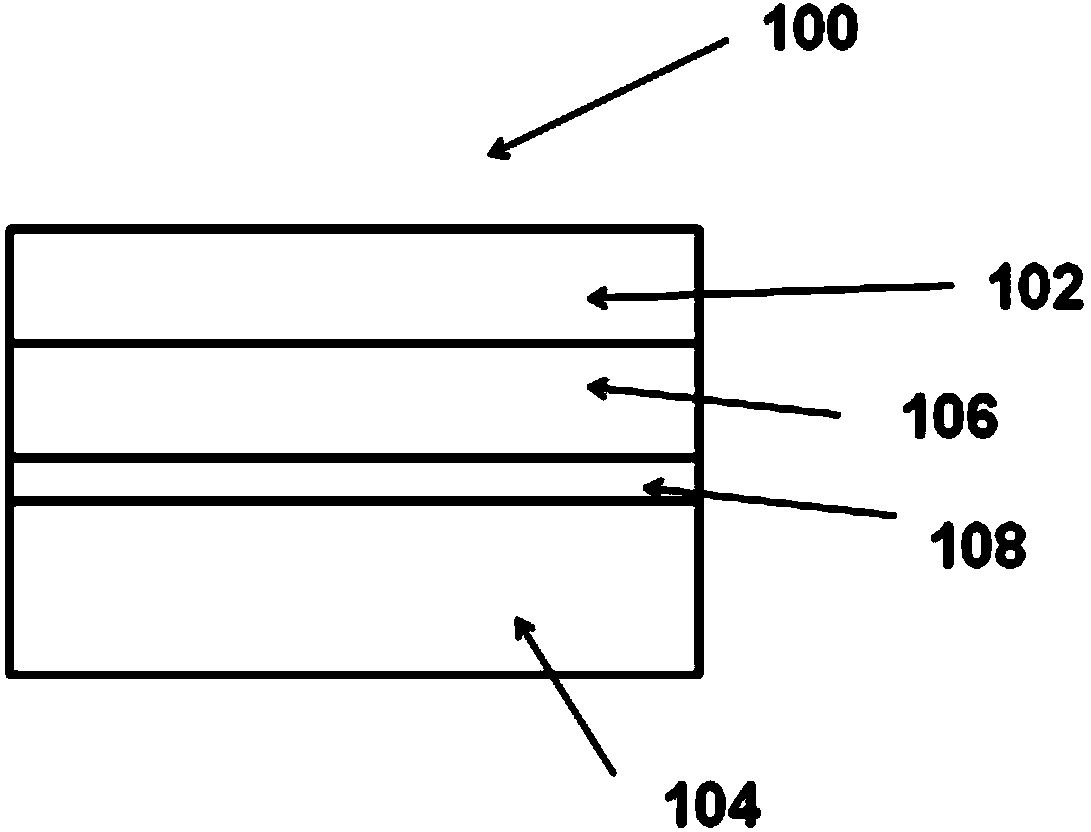
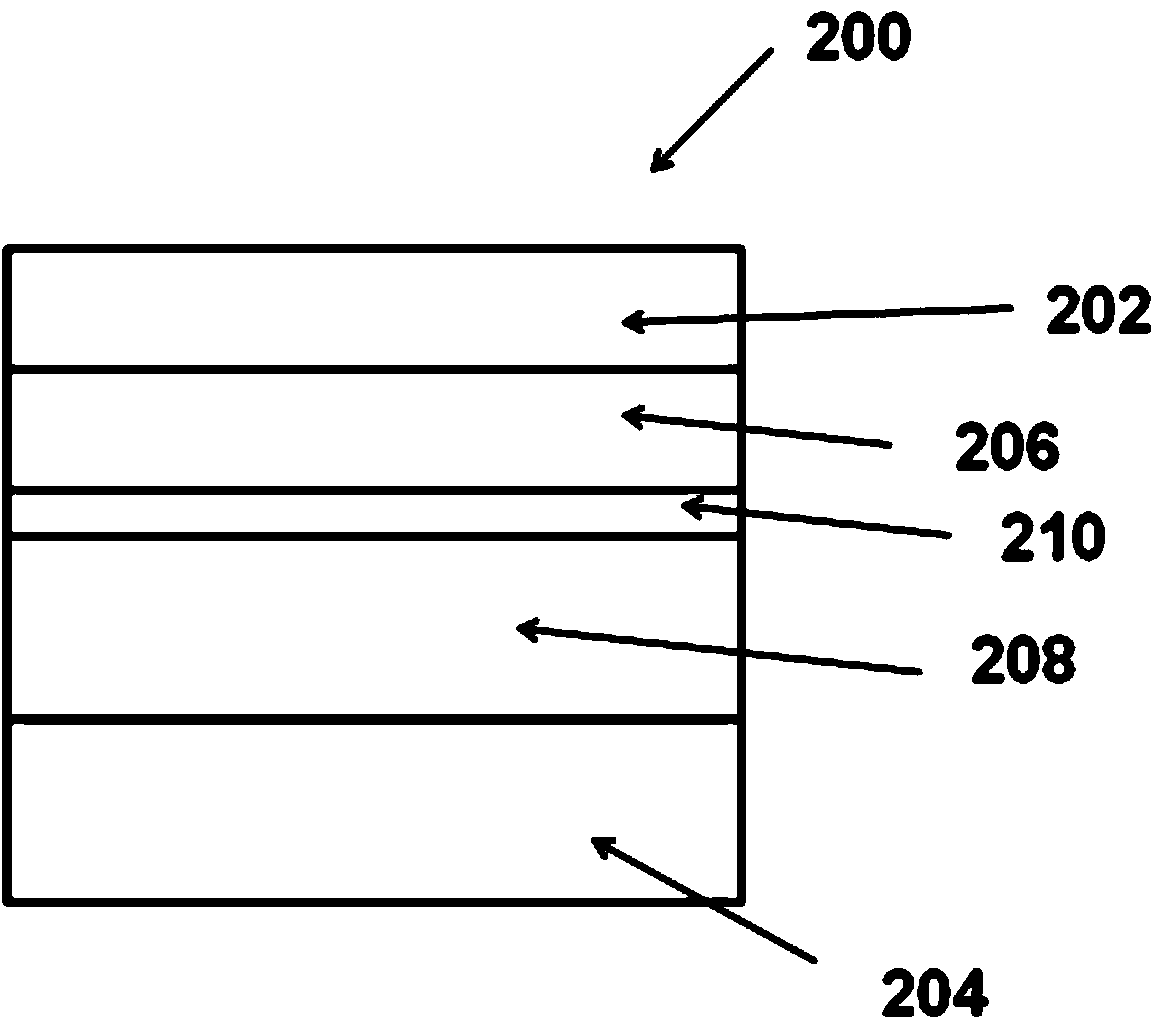
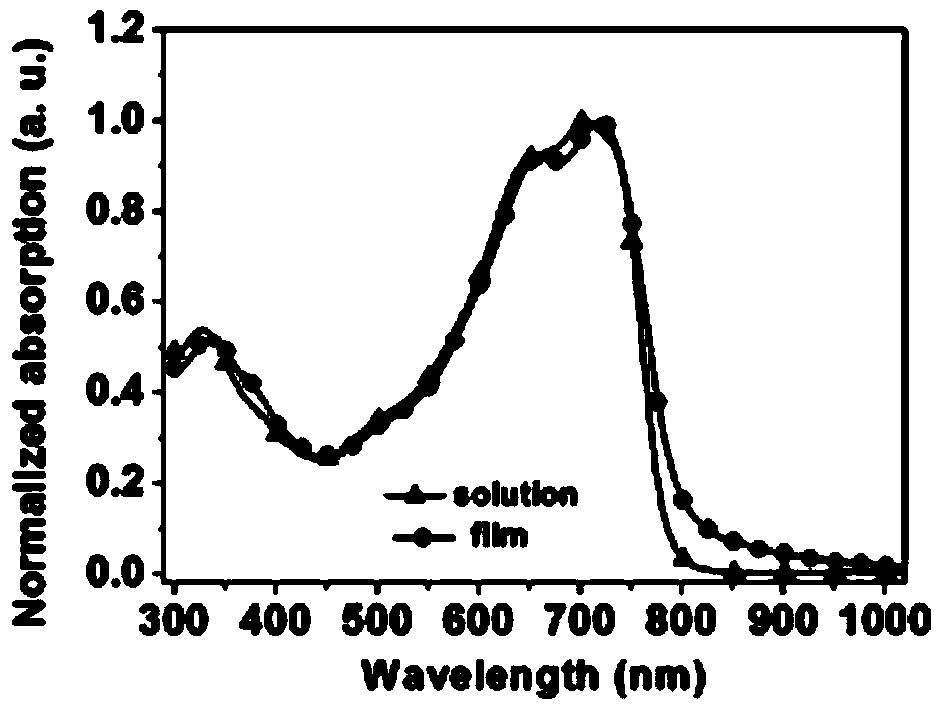
Image
Examples
Embodiment 1
[0103] Example 1.2,6-bis(trimethyltin group) 4,8-bis((2-ethylhexyl)thiothienyl)benzo[1,2-b:4,5-b']dithiophene ( M1) Synthesis of compounds
[0104]
[0105] Take 11.6g (0.1mol) of thiophene thiol and 20.7g (0.15mol) of potassium carbonate into a 250ml single-necked bottle, and dissolve with 100ml of DMF. Then take 19.3 g (0.1 mol) of 1-bromoisoctane with a syringe and add it to the reaction flask at one time. The reaction flask was placed in an oil bath at 80°C, and refluxed for 4 hours. Take the solution for GC-MS, it can be clearly seen that the product peaks at 8.5min. After the reaction was stopped, potassium carbonate was removed by suction filtration with a Buchner funnel, water was added to the filtrate, and extracted three times with ether. After the ether solvent was removed by rotary evaporation, the light yellow oily liquid product, 2-(2-ethylhexyl)thiophene sulfide (A01), was obtained by distillation under reduced pressure. (18.26g, yield: 80%)
[0106] The...
Embodiment 2
[0110] Example 2. Synthesis of Polymer P1
[0111]
[0112] Take 0.3mmol each of monomers M1 and M2 (purchased raw materials), dissolve them in a mixed solvent of toluene (8ML) and DMF (2ML), exhaust the air with argon for 5 minutes, and then add catalyst tetrakis (triphenylphosphine ) palladium(0) (20 mg) and continued to evacuate the air for 25 minutes, and then stop the polymerization after 14.5 hours at the reflux temperature of toluene. The polymer solution was cooled to room temperature, and slowly precipitated into methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hexane in a Soxhlet extractor. Finally, it was dissolved in chloroform, precipitated into methanol, filtered, and vacuum-dried for one day to obtain polymer P1 as a black solid powder. (49% yield) The structure of polymer P1 was confirmed by elemental analysis.
Embodiment 3
[0113] Example 3. Synthesis of Polymer P2
[0114]
[0115] Take 0.3mmol each of monomers M1 and M3 (purchased raw materials), dissolve them in a mixed solvent of toluene (8ML) and DMF (2ML), exhaust the air with argon for 5 minutes, and then add catalyst tetrakis (triphenylphosphine ) palladium(0) (20 mg) and continued to evacuate the air for 25 minutes, and then stop the polymerization after 14.5 hours at the reflux temperature of toluene. The polymer solution was cooled to room temperature, and slowly precipitated into methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hexane in a Soxhlet extractor. Finally, it was dissolved in chloroform, precipitated into methanol, filtered, and vacuum-dried for one day to obtain polymer P2 as a black solid powder. (41% yield)
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com