Aminopeptidase N inhibitor and preparation method and application thereof
An inhibitor, aminopeptidase technology, applied in the chemical field, can solve the problems of poor metabolic stability, weakening the ability of macrophages and NK cells to recognize and kill tumor cells, and decreased immunity of the body to achieve strong anti-tumor activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
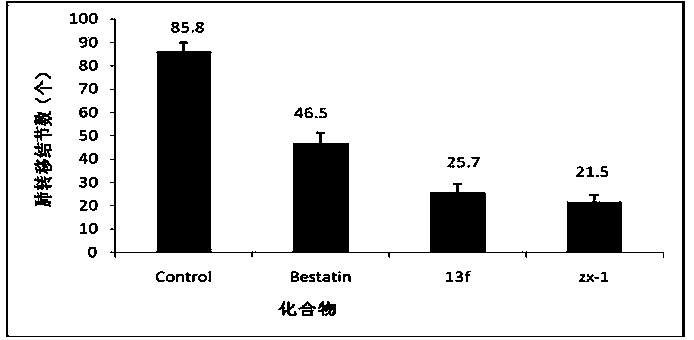
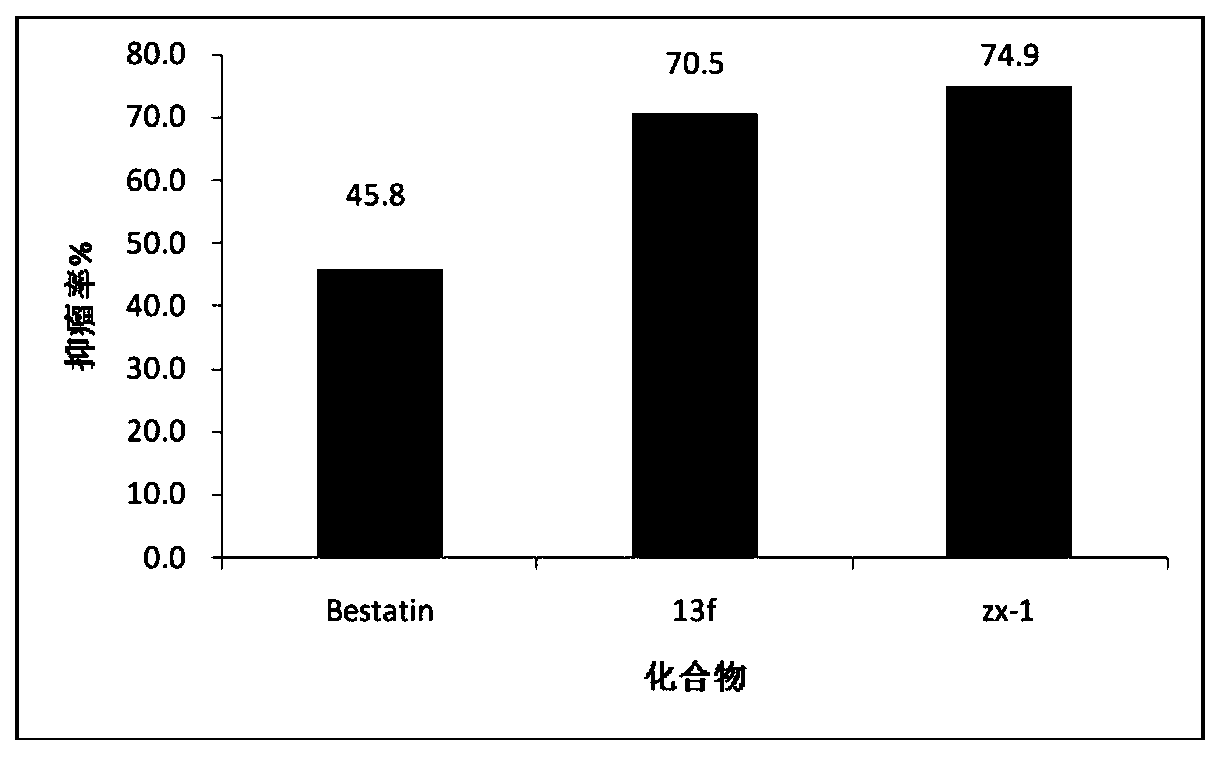
Image
Examples
Embodiment 1
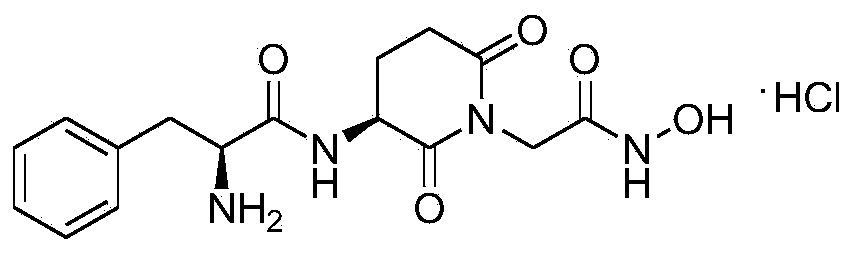
[0024] Embodiment 1. Synthesis of compound of the present invention (I)
[0025] 1) Preparation of (S)-tert-butyl-2,6-dioxopiperidin-3-ylcarbonamide (2)
[0026] Boc-L-glutamine (9.86g, 40.0mmol) and N-hydroxysuccinimide (4.6g, 40.0mmol) were dissolved in 100ml of tetrahydrofuran (THF), and 50ml containing di Cyclohexylcarbodiimide (8.24g, 40mmol) in THF. After about 1 hour of dripping, the ice bath was removed, and after stirring at room temperature for 3 hours, the reaction was refluxed for 10 hours. The reaction solution was cooled to room temperature, and the solvent was distilled off under reduced pressure. The residue was added to 40 ml of ethyl acetate and concentrated again. After the final residue was added with 50 ml of ethyl acetate, it was kept in the refrigerator overnight. Filter with 2.0g of diatomaceous earth, wash the filtrate with water (20ml×1) and saturated brine (20ml×1), dry over anhydrous magnesium sulfate, filter, and concentrate to dryness. Then r...
Embodiment 2
[0045] Example 2. Activity experiment of target compound inhibiting aminopeptidase N (In vitro)
[0046] The assay for aminopeptidase N (APN) inhibitory activity is described in Lejczak, B et al. Biochemistry, 1989, 28, 3549. The substrate L-leucyl-p-nitroaniline is degraded by APN to produce p-nitroaniline which absorbs at 405nm, and the concentration of p-nitroaniline is positively correlated with the enzyme activity. The content of p-nitroaniline is determined by detecting the absorbance at 405nm, so as to determine the activity of aminopeptidase, which indirectly reflects the degree of inhibition of the enzyme activity by the inhibitor.
[0047] For the specific method and operation steps, refer to CN100560568C "Cyclic imide peptide metalloprotease inhibitor and its application". The experimental results are shown in Table 1.
[0048] Table 1 In vitro enzyme inhibitory activity test results of target compounds
[0049]
[0050] The value in the table is the average v...
Embodiment 3
[0052] Example 3. The target compound inhibits tumor cell proliferation activity test (In vitro)
[0053]The in vitro anti-proliferation test of the compound on tumor cells was carried out using the thiazolium detection method (MTT method), and the cell suspensions of human ovarian cancer cell line (ES-2) or human breast cancer cell line (MDA-MB-231) were inoculated in In a 96-well plate, add medium containing different concentrations of compounds to each well, after incubation, stain with MTT, after continued incubation, measure the absorbance (OD value) of each well at 570nm on a microplate reader, and calculate the cell growth Inhibition rate to determine the activity of the compound.
[0054] 1. [Materials] ES-2 cell line, MDA-MB-231 cell line, tetramethylazoblue MTT, 10% fetal bovine serum, 96-well plate.
[0055] 2. [Method]
[0056] Cell culture The ovarian clear cell carcinoma ES-2 cell line and the breast cancer MDA-MB-231 cell line were cultured conventionally. Ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com