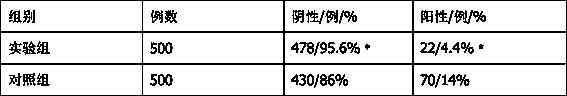
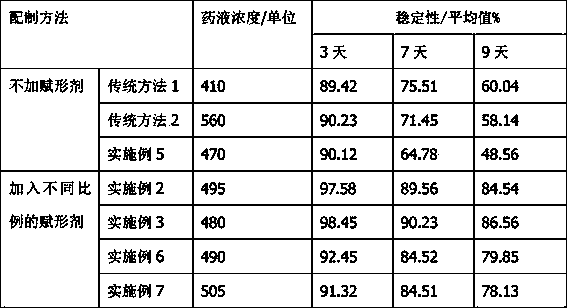
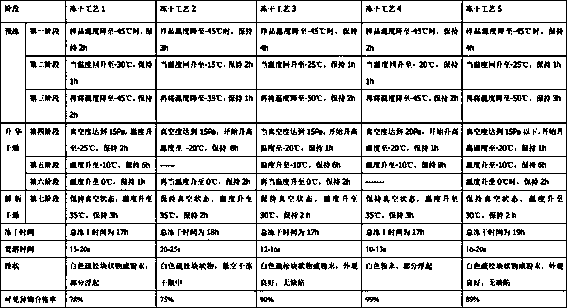
Novel penicillin skin test freeze-dried powder and preparation process thereof
The technology of freeze-dried powder and penicillin is applied in the field of new penicillin skin test freeze-dried powder and preparation technology, and can solve the problems of different types and contents of allergens, unstable penicillin-type water preparation, inaccurate concentration of skin test solution, etc. Achieve the effect of saving medical costs, good appearance and water solubility, and shortening freeze-drying time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This example describes the production of penicillin skin reagents containing 1000 units of penicillin sodium salt each.
[0038] Raw materials: 1.6 million units of penicillin sodium salt,
[0039] Excipients: Sodium Chloride 28.2 g
[0040] Composition of mannitol, dextran and lactose (the weight ratio of the three is 1:4.5:2) 85 g
[0041] Water for injection 3200 ml.
[0042] Penicillin sodium is mixed with sodium chloride and excipients, fully diluted with water for injection, and the diluted solution is divided into bottles containing 1000 units of penicillin salt, and sterilized by fine filtration through a 0.22 μm microporous membrane to obtain clarified penicillin sodium salt Solution, filter the liquid medicine into a sterile liquid storage bottle, check the clarity of the liquid medicine and the visible foreign matter (the same below), and aseptically pack 1ml of the above liquid medicine in a 5ml glass injection bottle. The sample is placed on ...
Embodiment 2
[0044] This example describes the production of penicillin skin reagents containing 2500 units of penicillin sodium salt each.
[0045] Raw materials: 1.6 million units of penicillin sodium salt,
[0046] Excipients: Sodium Chloride 5.8g
[0047] Composition of mannitol, dextran and lactose (the weight ratio of the three is 1:4.5:2) 36 g
[0048] Water for injection 640 ml.
[0049] After the above materials are fully mixed, each of the 5ml glass injection bottles is filled with 1ml of the above liquid medicine, and the divided samples are placed on the plate of the product chamber of the freeze dryer, and the condenser is heated at a speed of 20°C per hour. Pre-lower the temperature below -45°C. When the sample temperature drops to -45°C, keep it for 3 hours; when the temperature rises to -15°C, keep it for 2 hours; then lower the temperature to -35°C and keep it for 1 hour; Start vacuuming, when the vacuum degree reaches below 15Pa, start to raise the temperat...
Embodiment 3
[0051] This example describes the production of penicillin skin reagents containing 5000 units of penicillin sodium salt each.
[0052] Raw materials: 1.6 million units of penicillin sodium salt,
[0053] Excipients: Sodium Chloride 2.9 g
[0054] Composition of mannitol, dextran and lactose (the weight ratio of the three is 1:4.5:2) 17 g
[0055] Water for injection 320 ml.
[0056] After the above materials are fully mixed, each of the 5ml glass injection bottles is filled with 1ml of the above liquid medicine, and the divided samples are placed on the plate of the product chamber of the freeze dryer, and the condenser is placed at a speed of 25°C per hour. Pre-lower the temperature below -45°C. When the sample temperature drops to -45°C, keep it for 4h; when the temperature rises to -25°C, keep it for 1h; then lower the temperature to -50°C and keep it for 2h; after the sample is completely frozen, Start vacuuming, when the vacuum degree reaches below 15Pa, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com