Copolymer containing cyclopentadithiophene and benzodi(benzoselenadiazole), and preparation method and application thereof
A technology containing cyclopentadiene dithiophene and cyclopentadiene dithiophene, which is applied in the field of solar cell materials, can solve the problems of low photoelectric conversion efficiency and the like, and achieves advantages such as film forming processing, high yield and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
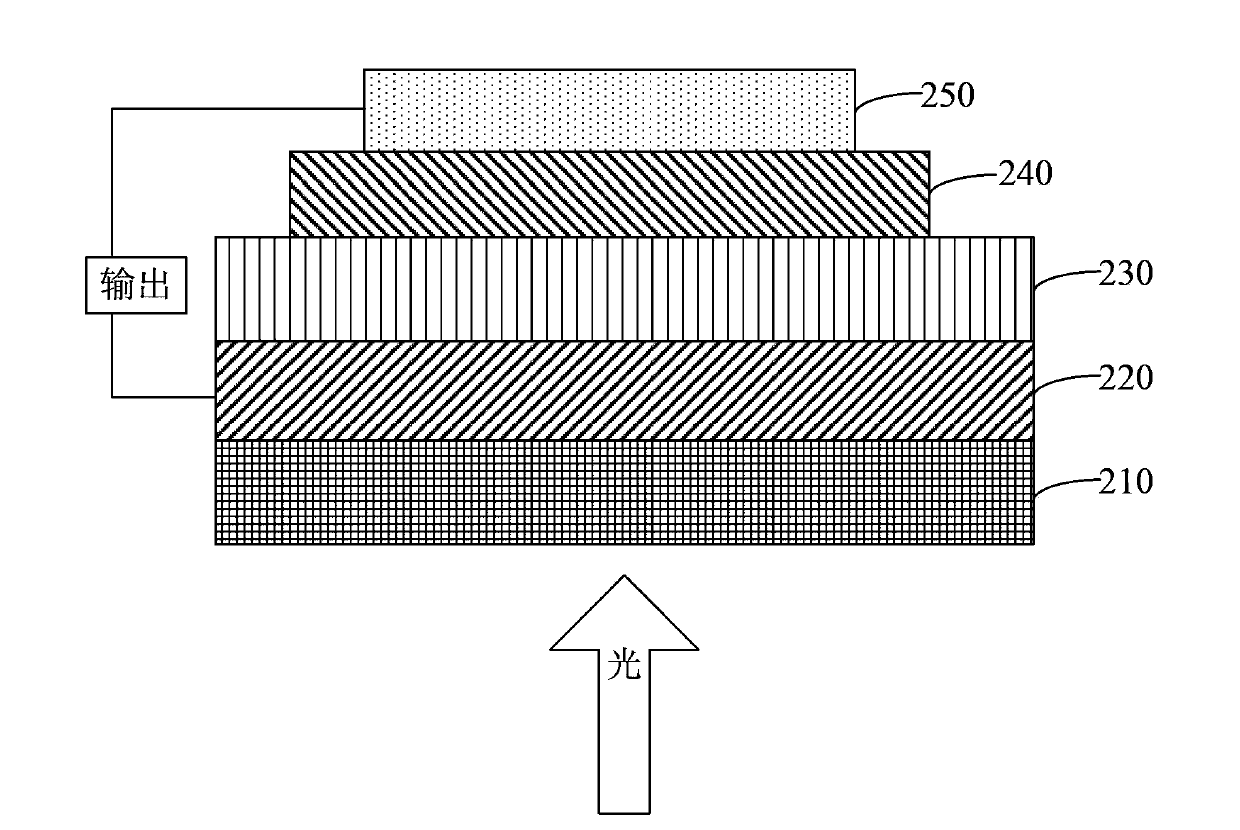
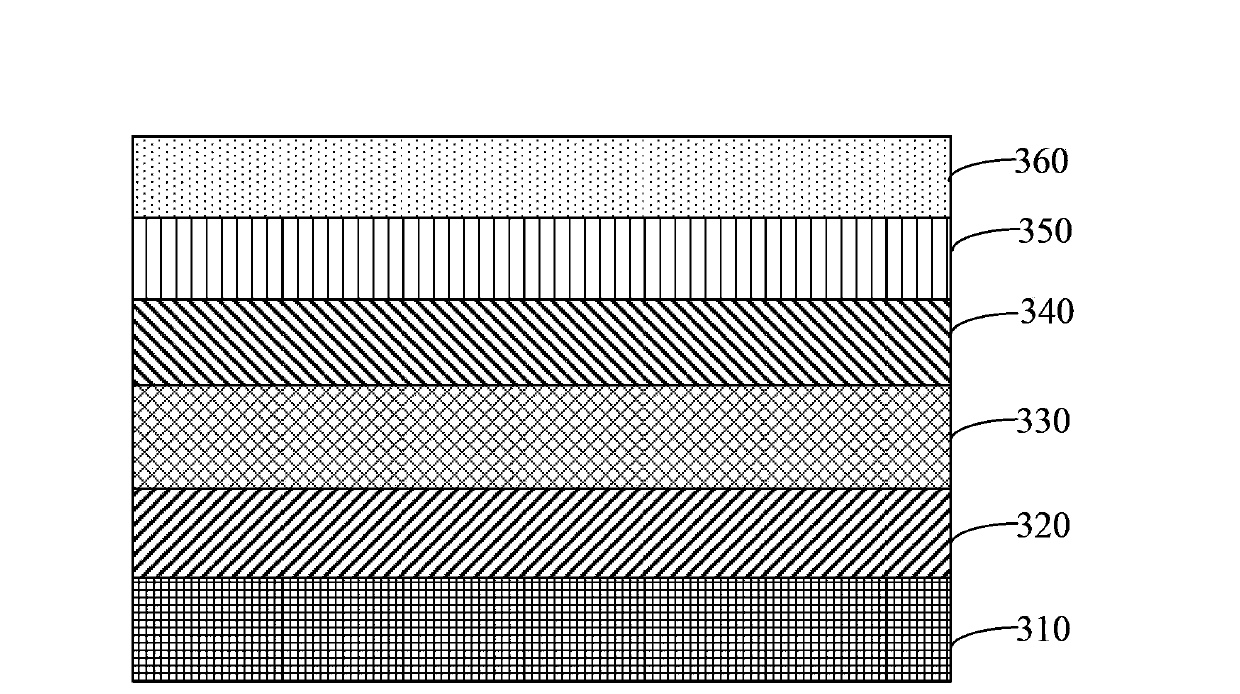
Image
Examples
preparation example Construction
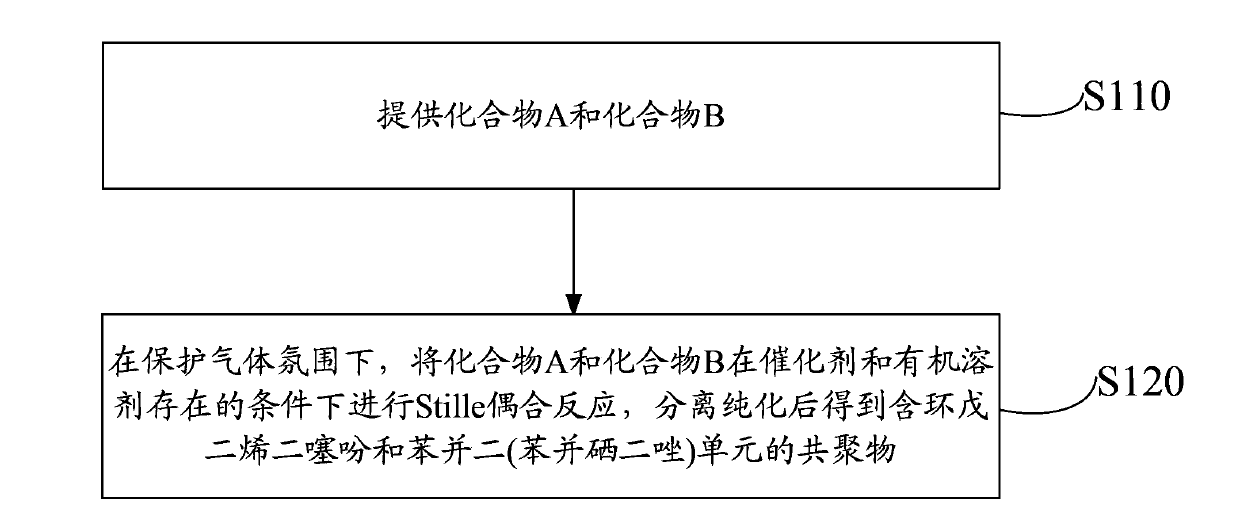
[0067] In addition, this embodiment also provides a preparation method of a copolymer containing cyclopentadiene dithiophene and benzobis(benzoselenodiazole) units, such as figure 1 shown, including the following steps:
[0068] Step S110, providing compound A and compound B. The structural formula of compound A is:
[0069]
[0070] The structural formula of compound B is:
[0071]
[0072] Among them, R 1 , R 2 , R 3 and R 4 for C 1 ~C 20 of alkyl.
[0073] Step S120, under a protective gas atmosphere, compound A and compound B are subjected to a Stille coupling reaction in the presence of a catalyst and an organic solvent at a molar ratio of 1:1.5 to 1.5:1, wherein the reaction temperature is 50 to 120 ℃, and the reaction time is 6 to 100 hours. After separation and purification, a copolymer containing cyclopentadiene dithiophene and benzobis(benzoselenadiazole) units with the following structural formula is obtained,
[0074]
[0075] Wherein, n is an in...
Embodiment 1
[0133] P1: Poly{4,4-dioctyl-cyclopentadieno[2,1-b;3,4-b']dithiophene-6,7-bis(3,7-dimethyl)octyl - benzo[2,1-e:3,4-e]bis(benzoselenodiazole)}, having the following structural formula:
[0134]
[0135] The preparation process of above-mentioned copolymer P1 is as follows:
[0136] Step 1, preparation of compound 5-nitro-2,1,3 benzothiadiazole
[0137]
[0138] Add 0.15 mol of 2-amino-5-nitroaniline and 100 mL of thionyl chloride into the three-necked flask, and slowly add 2 mL of pyridine dropwise to the three-necked flask under stirring conditions, and then heat and reflux for 24 hours; heat the reaction solution After the excess thionyl chloride is evaporated at 80°C, the reaction product cooled to room temperature is poured into a large amount of water and stirred; filtered to obtain a solid substance, and the solid substance after washing with water is vacuum-dried to obtain the product 5-nitro -2,1,3 benzothiadiazole 21.7g, yield 80%.
[0139] Step 2, preparation ...
Embodiment 2
[0165] P2: Poly{4,4-dimethyl-cyclopentadieno[2,1-b;3,4-b']dithiophene-6,7-bis(3,7-dimethyl)octyl - benzo[2,1-e:3,4-e]bis(benzoselenodiazole)}, having the following structural formula:
[0166]
[0167] The preparation process of above-mentioned copolymer P2 is as follows:
[0168] Steps 1-7 are the same as Steps 1-7 of Example 1.
[0169] Step 8. Preparation of 4,4-dimethyl-2,6-bis(tributyltin)-cyclopentadieno[2,1-b;3,4-b']dithiophene
[0170]
[0171] Under the protection of nitrogen, add 0.01mol 4,4-dimethyl-2,6-dibromocyclopentadiene[2,1-b;3,4-b']dithiophene and 40ml tetrahydrofuran into the three-necked flask solvent to obtain a mixed solution; slowly inject 8.6mL of n-butyllithium (0.02mol) into the mixed solution with a syringe under the condition of -78°C, and continue to stir and react for 1.5 hours; Inject 5.6mL of tributyltin chloride (0.02mol) into the solution, stir for 1 hour, then warm up to room temperature and continue to stir for 6 hours; add 30ml of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com